Geology and Natural Heritage of the Long Valley Caldera
Hydrothermal Alteration and Mineral Deposits in the Sierra Nevada Mountains
Crystal Chen
Abstract
Hydrothermal alteration has had an enormous impact on the shaping of the Sierra Nevada, leading to a wide variety of mineral deposits all along the mountain range, including the famous gold deposits of the California Gold Rush. Although acid-sulfate alteration and adularia-sericite alteration, two types of hydrothermal alteration, have common features and share a similar volcanic environment, they are visibly distinct and differ in mineral composition. Distinguishing between these processes is important for determining the mineralogy and potential epithermal nature of a hydrothermal mineral deposit.
Introduction
The California Gold Rush from 1848 to 1855 drew hundreds of thousands of people from twelve different countries to California in a desperate search for gold, amounting to the largest migration to the United States in its history. Migrant miners extracted astonishing amounts of gold from the ground. In 1852 alone, $81 million worth of gold was discovered by miners from all across California. Not only did the California Gold Rush unearth millions of dollars for lucky miners, but it also unearthed massive amounts of bedrock from the Sierra Nevada [Longsworth and Pachter, 2006]. Over the span of the gold rush, hydraulic miners removed 40 billion cubic feet of Sierra Nevada bedrock. In relation to other large scale earth-moving operations, hydraulic miners excavated about five times more earth than that of the Panama Canal excavation [Meldahl, 2011]. Because relatively little was known about gold mining and the geology of the western United States at the time, the California Gold Rush provided a wealth of information about the eastern Sierra Nevada and the various processes that led to the precipitation and accessibility of gold through the excavation of large amounts of land and observations made by thousands of miners. Ultimately, the California Gold Rush played a crucial role in the examination of the role of hydrothermal alteration and hydrothermal mineral deposits in the eastern Sierra Nevada region [Longsworth and Pachter, 2006].
Hydrothermal alteration is a common scientific phenomenon concerning the interaction between hot water in the Earth’s crust and the surrounding rock, where hot water, steam, or gas create changes in the chemical and thermal environment of the rock. These environmental changes subsequently trigger chemical, mineralogical, or textural responses in the rock. Mineralogical responses include changes in the minerals’ properties, distribution, and identification [Wohletz and Heiken, 1992]. Hydrothermal alteration is a very dynamic earthly process, where the change it wreaks is itself changing due to varying pressures, temperatures, mineral stabilities and chemical saturations. However, water always serves as the main force that concentrates, transports, and deposits minerals, which are either present in the original igneous source of water or are leached from surrounding rock as the water travels [Skinner, 2006].
While hydrothermal alteration occurs in a wide array of geologic conditions, it is especially common around fault zones and explosive volcanic features. Thus, the eastern Sierra Nevada harbors very ideal conditions for hydrothermal alteration, featuring hydrothermal mineral deposits that vary greatly in age and composition but that all formed through the same general series of steps [Hill and Bailey, 1985]. First, there must be water that is hot enough to dissolve and carry the minerals from the magma chamber to the Earth’s surface. Second, there must be a network of fractures and porous areas in the rock, serving as a passageway for the solution to move through. Third, there must be available areas for the water to dispose of the minerals in a process known as deposition. Fourth, there must be a chemical reaction preceding deposition [Encyclopedia Britannica hydrothermal mineral deposit website, 2014].
There are two different types of hydrothermal alteration, namely acid-sulfate and adularia-sericite alteration. The first consists of waters with high sulfide content whereas the latter consists of waters with low sulfide content. These two types of hydrothermal alteration have several similar components, but they feature key differences in the origin of the hydrothermal fluids and the composition and appearance of its hydrothermal mineral deposits. More specifically, the hydrothermal fluids equilibrate with their host rocks at different depths, which changes the characteristics and influence of the hydrothermal fluid when it interacts with rocks at shallower depths. Consequently, the two processes differ in the parts of the system that give rise to economic mineralization. The resulting mineral deposits have different forms, mineralogy, texture, and distributions of alteration zones. Thus, it is important to distinguish between the two hydrothermal alteration processes when searching for and examining hydrothermal mineral deposits [Hedenquist, 1995].
History of Sierra Nevada mountain range
The Sierra Nevada mountain range began to form 20 million years ago due to plate tectonics. Previously, the Sierra Nevada Mountains resided beneath the sea, accumulating sediment from the North American continent on the North American Plate to the east. When the Pacific Plate subducted beneath the North American Plate, the subduction forces melted the sediment and created a colossal magma chamber. Over millions of years, the magma chamber slowly cooled into granite. Due to the principle of isostasy and subduction forces, the granite uplifted and formed an enormous mountain range above sea level, forming the basis of the Sierra Nevada mountain range seen today [Sally Ride Science Investigating Sierra Nevada website, 2015]. The magma’s composition continued to morph with recent eruptions, which shifted from mostly basaltic eruptions to mostly rhyolitic eruptions. The change in the type of eruption led the igneous magma to become more silica-rich over time. Rhyodacite intrusions, which are silica-rich, intruded into these older volcanic formations through cracks and pores in the rock. Because these intrusions had high levels of hot and salty water, the mineral elements were able to evolve out of the rock when the intrusions reached the Earth’s surface [USGS Long Valley website, 2012].
There are two main groups of hydrothermal mineral deposits, specifically containing gold, in the Sierra Nevada Mountains. The Sierra Nevada Batholith gold deposits are only found on the western side of the Sierra Nevada mountain range and formed 175 million years ago. These deposits were the destination of the famous 1849 Gold Rush and mostly consist of quartz veins with traces of gold that wove up through the granite and precipitated out into the metasedimentary rocks atop the granitic batholith. The other main set of gold deposits are the 25 million year-old epithermal deposits primarily located in Bodie, California, Goldfield, Nevada and Creede, Colorado. An epithermal deposit has a lower temperature relative to other deposit types and is typically a rich conglomeration of various precious metals, thus deserving of the name "bonanza." Both of these deposits formed through hydrothermal alteration [Bailey, 1976].
Hydrothermal solutions
Characteristics
In the eastern Sierra Nevada, extremely hot waters varying from 50 to 300 degrees Celsius circulate above the magma chambers of an igneous intrusion. The water comes from various sources and creates a hydrothermal solution when water and dissolved minerals from different sources converge. The water could be in the form of ocean water or rainfall and travel great depths from the Earth’s crust, heating as it travels deeper and deeper within the Earth and reacting with the rocks it passes. In addition, rocks undergoing metamorphism often release water, as do magma when it crystallizes. Hot water originating from the magma chamber often comes into contact with dissolved minerals from the magma’s soluble components. When this hot water intermixes with the cooler, circulating groundwater, a reaction occurs that releases ore parts, and a hydrothermal solution is formed [Skinner, 2006].
Hydrothermal solutions are able to sweep along minerals because of their extreme heat and high salt concentrations. This is because they consist of natural, hot brines rather than pure water. Natural brines are highly concentrated solutions of water and sodium chloride, thus serving as a vital resource of many salts, including potassium and magnesium sulfates and chlorides. Due to its high levels of salt, hydrothermal solutions are efficient solvents of most ore minerals, which contain a lot of sulfide and oxide. In addition, hydrothermal solutions have the ability to dissolve and easily transport metals such as silver and gold [Skinner, 2006].
Pathway
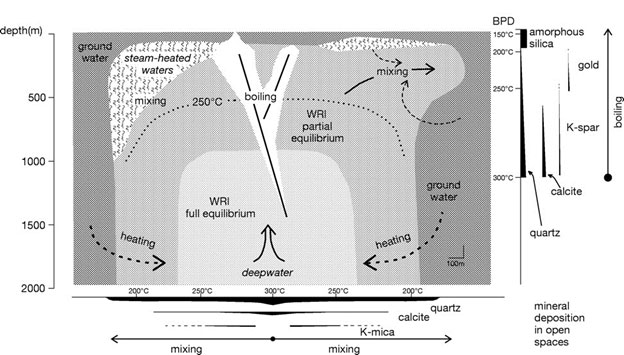
After the hydrothermal solution is formed, it then flows through the path of least resistance through porous areas or cracks in the bedrock, known as fractures or joints. The pathways traveled by hydrothermal solutions are often changing due to shifting tectonic plates and earthquake faulting. As the solution approaches the Earth’s surface, it rapidly loses heat. This cooling occurs because of the geothermal gradient, which illustrates the direct relationship between heat in the Earth and depth. Generally, the geothermal gradient occurs at a rate of about a two-degree Celsius increase with every 100 meter increase in depth, but in areas near tectonic plate boundaries or volcanic activity, such as the eastern Sierra Nevada, the geothermal gradient rate is drastically higher due to the close proximity of heat originating from the magma chamber, as well as due to the movement of magma itself. In these areas, water is often at or above the boiling point of 100 degrees Celsius, yet it remains in the liquid state due to the overwhelming pressure from overlying rock. This "superheated water" is present at very shallow depths, which gives it the potential to continue reacting with surrounding minerals embedded in the bedrock before it completely cools down [Dumitru, 2012].
Figure 1. This diagram shows the pathway of hydrothermal fluids and the main hydrothermal alteration processes within the top 2 kilometers from the Earth’s surface [Dumitru, 2012].
Hydrothermal mineral deposits
When the hydrothermal solution approaches the Earth’s surface, it deposits the dissolved minerals into the rock. This happens for a variety of reasons. When the hydrothermal solution reaches shallower depths, the decrease in temperature could cause the minerals to precipitate out, as different minerals have different crystallization temperatures. Other changes leading to deposition include boiling and chemical reactions between the hydrothermal solution and the surrounding bedrock [Skinner, 2006].
The minerals will separate from the rest of the hydrothermal solution through two different processes. They may precipitate out into open fractures in the rock and form a nearly pure vein of ore, which has definite boundaries in the midst of "gangue" minerals, or non-ore minerals. Otherwise, the hydrothermal solution will disseminate into surrounding bedrock. Before the hydrothermal solution cools amongst the bedrock, it dissolves the rock and reacts with minerals in the surrounding rock that commonly fill veins, such as calcite or quartz. The solution ultimately deposits a new assemblage of gangue minerals in the original rock’s place. This is known as a replacement deposit [Encyclopedia Britannica hydrothermal mineral deposit website, 2014].
These two deposition processes often take place at the same time, so hydrothermal solutions will fill in openings in the rock through precipitation while simultaneously replacing the walls around the fracture [Skinner, 2006]. Deposition increases the bulk density of the rock, which is calculated by the mass of accumulated rock particles divided by the total volume occupied by the rock, and decreases the rock’s permeability and porosity [Wohletz and Heiken, 1992].
When hydrothermal deposits form near a boiling hot spring system within 1 kilometer of the Earth’s surface between 50 to 200 degrees Celsius, then the deposit is referred to as an epithermal deposit [Wohletz and Heiken, 1992]. These deposits are considered bonanzas and although they only reach shallow depths, they contain a rich variety of minerals. Epithermal deposits are often more colorful than rocks that did not undergo hydrothermal alteration due to the various chemical reactions between different types of rocks [Skinner, 2006].
Types of hydrothermal alteration
Origin of hydrothermal fluids
The two different types of hydrothermal alteration are acid-sulfate alteration, where the hydrothermal solution has high levels of sulfides, and adularia-sericite alteration, where the hydrothermal solution has low levels of sulfides. These two fluids differ in their source and the depth at which they equilibrate with their host rocks, which leads to their different compositions [Hedenquist, 1995].
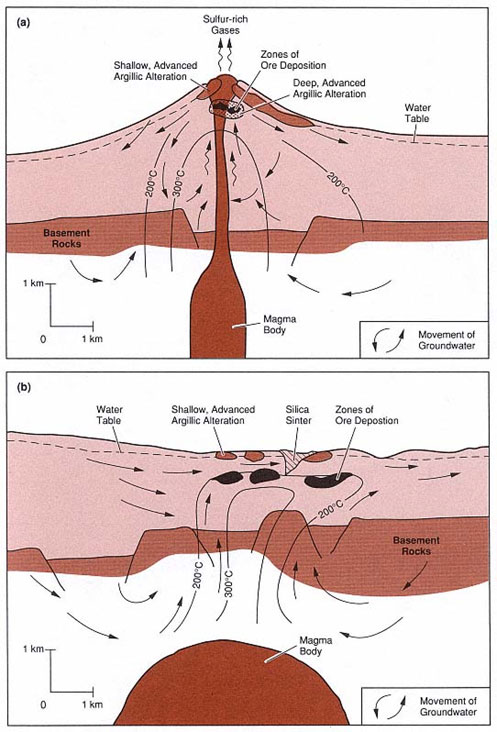
In acid-sulfate alteration, groundwater mixes with HCl and SO2 gases from the magma, creating acid-sulfate water. Thus, the hydrothermal solution is very oxidized and acidic. This system requires large amounts of cool groundwater that retains its original chemical characteristics and originates from within 1 kilometer of Earth’s surface. There is very little water-rock interaction except at very shallow depths, where the hydrothermal fluid leaches minerals from the host rock into its fluid and ultimately creates replacement deposits. Thus, the fluid equilibrates with the host rock near the end of the hydrothermal alteration process. This system is common along caldera ring fractures or near the uppermost parts of young volcanoes, because these locations harbor a magmatic-hydrothermal environment [Hedenquist, 1995].
Figure 2. This model shows two types of hydrothermal alteration. (a) In the figure depicting acid-sulfate alteration, the arrows represent magmatic gases that condense and oxidize to form acid-sulfate fluids. (b) In the figure depicting adularia-sericite alteration, the arrows represent alkali-chloride, neutral pH waters [Wohletz and Heiken, 1992].
Contrastingly, in adularia-sericite alteration, the hydrothermal fluids have a neutral pH and contain high levels of alkali chlorides. Relatively, adularia-sericite alteration fluids are cooler and less acidic at 100 degrees Celsius with a pH ranging from 2 to 3. These fluids equilibrate with their host rock much earlier in the hydrothermal alteration process and are common in active geothermal systems. They may also originate from hot springs with a pH that is generally neutral. Because the fluids are in equilibrium with their host rock at great depths, NaCl, CO2, and H2S are very common elements. The hydrothermal solution rises through fractures and deposits ore and gangue minerals. When the hydrothermal solution reaches the boiling point at shallow depths, a CO2 and H2S rich vapor condenses and forms very hot acid-sulfate waters after H2S oxidizes. For most of this process, until about 1 to 2 kilometers from the Earth’s surface, the alkali-chloride waters retain a neutral pH. In conclusion, the two systems’ fluids have very different characteristics at varying depths [Hedenquist, 1995].
Forms of Deposit
In acid-sulfate alteration, veins are important for collections of ore, but the majority of ore is disseminated in surrounding bedrock and in replacement deposits because the high acidity of the hydrothermal solution contributes to intense leaching of the rocks lining the walls of fractures. Conversely, in adularia-sericite alteration, the ores accumulate in veins with distinct boundaries separating the veins from the surrounding gangue minerals. There is relatively less dissemination in this form of alteration because the hydrothermal fluid has a near neutral pH, so it does not dissolve the surrounding rock as easily. Although both forms of alterations are usually structurally controlled, this trait is more difficult to identify in acid-sulfate alteration because it is covered up by the disseminated ores.
Overall, ore in acid-sulfate alteration deposits is closely related to the zone with the highest amounts of acid alteration, while ore in adularia-sericite alteration deposits is associated with the zone with the lease amounts of acid alteration. This is all due to the differing pH levels of the hydrothermal fluid. If the adularia-sericite deposit contains acidic alteration, there are surface factors to consider. For instance, acid-sulfate waters heated by steam from hot springs could have participated in alteration, or there may be post-hydrothermal erosion of sulfide minerals [Hedenquist, 1995].
Mineralogy
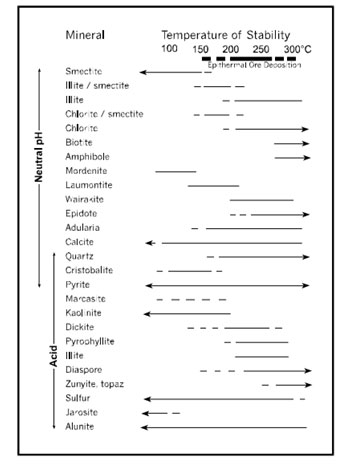
The minerals deposited from both types of hydrothermal alteration maintain considerable overlap, but the sulfide mineralogy varies due to different redox conditions, as acid-sulfate fluids are more oxidized compared to adularia-sericite fluids. In acid-sulfate alteration, copper is very common, as are other high-sulfidation state minerals such as tennantite-tetrahedrite. These types of minerals are very rarely seen in adularia-sericite alteration deposits, which more often feature minerals such as sphalerite and arsenopyrite. Gangue minerals also convey some variability because of the hydrothermal fluids’ different pH levels, which directly relates to the level of reactivity between the hydrothermal fluid and the surrounding rock. Acid-sulfate deposits feature minerals such as diaspore, pyrophyllite, alunite, kaolinite, and other minerals that require somewhat acidic conditions to form. Calcite, adularia, and other minerals requiring near-neutral pH levels to form are found in adularia-sericite deposits. However, both types of deposits commonly feature quartz, which can form under a wider range of conditions [Hedenquist, 1995].
Figure 3. This diagram depicts the various temperatures at which common hydrothermal minerals in epithermal deposits achieve stability. In acid-sulfate alteration deposits, which are high-sulfidation systems, the main gangue mineral is quartz. In adularia-sericite alteration deposits, which are low-sulfidation systems, the main gangue minerals are calcite, adularia and quartz [Hedenquist, 1995].
Gold precipitation also occurs through two different methods. In adularia-sericite alteration deposits, gold originally exists as a bisulfide complex because it is surrounded by a reduced fluid with very low levels of salinity. When the hydrothermal fluid is about 1 to 2 kilometers from the Earth’s surface, CO2 is released from the liquid through boiling. The resulting increase in pH level at first increases the gold’s solubility, but this change is reversed when the accompanying H2S loss decreases gold’s solubility and allows it to precipitate. In contrast, in acid-sulfate alteration deposits, gold exists in the hydrothermal fluid as a chloride complex with many different influences on when it precipitates out of the fluid [Drummond and Ohmoto, 1985].
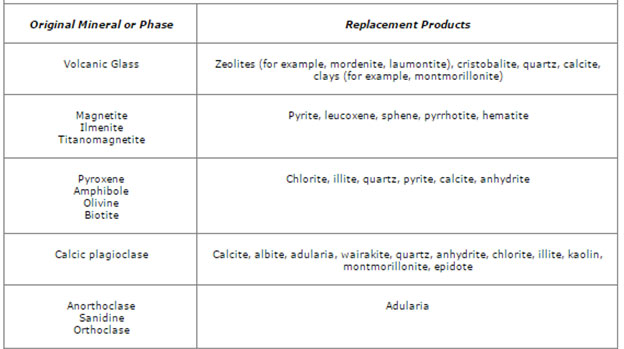
Figure 4. This table shows typical replacement products from both types of hydrothermal alteration, namely acid-sulfate alteration and adularia-sericite alteration. This shows that hydrothermal fluids react with the original mineral and subsequently deposit a new mineral product [Hedenquist, 1995].
Texture
Perhaps the most visible indicator of which type of process a hydrothermal mineral deposit underwent would be its texture. Acid-sulfate deposits have very little variation in its texture and are most distinguishably characterized by large bodies of vuggy quartz. A vug is a small or medium cavity inside a rock that is filled with minerals. Vuggy quartz is caused by the acidic hydrothermal fluids’ leaching, which extracts minerals from the host rock and leaves behind openings. These fractures are filled with the remaining silica, which recrystallizes to quartz when the rock retains the temperature it was at before heating up from the hydrothermal fluid [Hedenquist, 1995].
In contrast, a wide variety of textures can be found in adularia-sericite alteration deposits because they are typically a conglomeration of precious metals. These ore deposits often are layered in a complicated, overlaying pattern due to multiple cycles of varying intensities of hydrothermal alteration. Multiple-episode veins are also molded by a release of explosive pressure from hydrothermal eruptions. If the deposit has undergone very little erosion, then silica sinters deposited from hot spring waters are visible [Hedenquist, 1995]. Silica sinters are identifiable by their rhythmic bands, fine-grained matrix, and embedded opal and quartz crystals. Plant fragments can occasionally be found within silica sinters as well [Imperial College London Silica Sinter website, 2013]. Kinetic factors allow for the polymerization and precipitation of silica from the near-neutral solution, thus resulting in the high levels of silica in these deposits [Hedenquist, 1995].
Conclusion
We have briefly discussed the formation of the Sierra Nevada mountain range and how it is an ideal environment for hydrothermal alteration, as well as the general process behind hydrothermal alteration and its properties that allow for mineral deposits. The two kinds of hydrothermal alteration in volcanic settings have distinct textures and deposit forms that are easily identifiable in the field, and the processes vary greatly in hydrothermal fluid properties and the reactions along the water pathway, though both processes share a similar environment. Distinguishing between the two forms of hydrothermal alteration is important when identifying whether a deposit is epithermal. This information will prove valuable for future exploration into hydrothermal mineral deposits and during the hunt for epithermal ore deposits.
List of References
Bailey, Roy A. Volcanism, Structure and Geochronology of Long Valley Caldera, Mono County, California. Journal of Geophysical Research 81.5 (1976): 732. Web. 16 June 2015.
Drummond, S.E., and Ohmoto, H. Chemical Evolution and Mineral Deposition in Boiling Hydrothermal Systems. Economic Geology Society of Economic Geologists, 1 Feb. 1985. Web. 17 June 2015.
Dumitru, Trevor A. Subnormal Cenozoic Geothermal Gradients in the Extinct Sierra Nevada Magmatic Arc. Solid Earth. Journal of Geophysical Research, 20 Sept. 2012. Web. 17 June 2015.
Hedenquist, J. Epithermal Gold Deposits: Styles, Characteristics and Exploration. SEG Newsletter 23 (1995): 1+. ResearchGate. Web. 14 June 2015.
Hill, David P., and Bailey, R.A. Active Tectonic and Magmatic Processes Beneath Long Valley Caldera, Eastern California: An Overview. Journal of Geophysical Research 90.13 (1985): 116-17. Web. 12 June 2015.
Hydrothermal Mineral Deposit. Geology. Encyclopedia Britannica, 19 Feb. 2014. Web. 13 June 2015.
Investigating The Sierra Nevada Mountains and Lake Tahoe. Sally Ride EarthKAM. Sally Ride Science, 2015. Web. 15 June 2015.
Long Valley-Mono Basin Region. Geologic History of the Long Valley, Mono Basin Region. USGS, 6 Feb. 2012. Web. 15 June 2015.
Longsworth, L., and Pachter, A. California Gold Rush. The Gold Rush. PBS, 13 Sept. 2006. Web. 13 June 2015.
Meldahl, K.M. Wealth and Magma. Rough-Hewn Land. 1st ed. Oakland: U of California, 2011. 117-30. Print.
Silica S. Rock Library. Imperial College London, 2013. Web. 18 June 2015.
Skinner, B.J. Hydrothermal Solution. Mineral Deposit. Encyclopedia Britannica, 2 Aug. 2006. Web. 12 June 2015.
Wohletz, K., and Heiken, G. Surface Manifestations of Geothermal Systems. Volcanology and Geothermal Energy. Berkeley: U of California, 1992. 130-41. Print.
[Return to Research Projects] [Return to Sierra Home]