Geology and Natural Heritage of the Long Valley Caldera
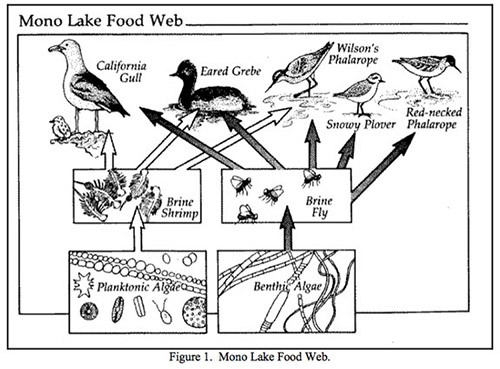
Mono Lake Ecology
Alex Hughes
Abstract
The creation of Mono Lake has given rise to an ecosystem unlike any other. This paper ranges from microscopic organisms to millions of birds and the human influence. Each piece below has a significant role in the area’s ecology.
Geological Factors
The warping of Earth’s crust and faulting to the east of the Sierra Nevada mountain range created a basin over 760,000 years ago. The basin fell eleven thousand feet below its original surface level. Gravity forced water from above to cut its way into the landscape in search of lower ground as glaciers and seasonal snows melted. After the water crashed its way from the tops of mountains, it gathered at the low point that became Mono Lake. Since the lake is the lowest ground, there are no outlets to carry the water and sediment materials any further. Over time, four thousand feet of the original depth of the basin filled with sediments from streams (Hill, 2006). When water enters the lake, it is left simply to dissipate. At an evaporative rate of four vertical feet per year, the constant supply of water from the mountains has kept Mono Lake in existence.
Lake Conditions and Chemistry
The lack of an outlet leaves the lake with unique chemical properties. Mono Lake has salinity two to three times that of the ocean (Mono Lake Committee Website). Hydrothermal vents in the lake and periodic volcanic eruptions add to the chemical properties of the lake (Oremland et al., 2002). At a pH level of 9.8, Mono Lake is eighty times as alkaline as the ocean and noted as "The dead sea of The West" by Mark Twain (Humayoun et al., 2003)(Lawrence, 1985).
Extremophiles
The conditions of Mono Lake do not support any species of fish. To appreciate the diversity of aquatic organisms in Mono Lake it is necessary to observe life at a microscopic scale. Microbes known as extremophiles have evolved to withstand the harsh conditions of the lake. Occasionally, high winds will cause internal waves in Mono Lake to churn the nutrients (MacIntyre et al., 1999). However, for almost two decades the lake has slowly been adjusting from a meromictic state. The decrease of vertical nutrient flux in Mono Lake was due to the rapidly increased levels of flow to Mono Lake during the mid–1990’s. It is assumed that the microbial ecosystem was altered by this drastic change. However, the majority of data regarding the bacteria and archaeans of Mono Lake was collected after this time period. Studies have shown the correlation of depth and organism type based on maintained niches in depths containing specific nutrients or gases.
Biologically reduced substances accumulate at the greatest depths. At these depths, elevated levels of ammonia and sulfide were originally hypothesized to decrease the amount of bacteria that could survive the anoxic environment. However, a study published in Applied and Environmental Microbiology in 2003 indicates that bacteria living near the bottom of Mono Lake display a higher level of biological diversity than those existing in the upper portions. In the bottom portion of Mono Lake, the chemical components are relatively stable throughout (Humayoun et al., 2003). Therefore, it has been hypothesized that the diversity is not due solely to the chemo–physiology of the water in this layer, but to the lack of competition of other microbes. Bacteria with oxygen as the basis of respiration would not out–compete the less–efficient anaerobic respiration of the extremophiles. The result is an ecosystem that is not limited to a few organisms battling for survival, but many anaerobic variants with a lack of aerobic competition (Madrid et al., 2001). However, a study involving samples at surface, midlevel, and the bottom of Mono Lake indicates that the highest concentrations of viruses are found at the greatest depths. Viruses are fundamental portions of aquatic ecosystems and are key sources of bacterial mortality. The variation of viruses in vertical and horizontal fields may shed light on the increased biodiversity of extremophiles at greater depths (Jiang et al., 2004).
Benthic Algae
Benthic algae also occupy the greater depths of Mono Lake. The optimum conditions for growth include sand and organic rich mud sediment. At temperatures of only one to six degrees Celsius, the algae thrive. Benthic algae absorb the small amounts of sunlight that make it to the floor of Mono Lake. Here, the multicellular algae form cohesive microbial mats. These are mostly composed of the filamentous blue-green algae and diatoms (Mono Lake Committee Website). Over thirty taxa of benthic algae are recorded from Mono Lake at samples varying from one to ten meters (Herbst and Bradley, 1989). These algae provide the nutrients for the lake’s most popular insect.
Alkaline Fly
The alkaline fly of Mono Lake, Ephydra hydropyrus hians, begins its life as an egg on the benthic algal mat. Once hatched, the larva grows very quickly. Before becoming fully grown larvae, the young alkali–fly sheds its skin two times (Simeone, 2001). The shedding is part of the larva’s growth, divided into three phases called instars (Herbst, 1990b). It may take anywhere from four weeks to five months for the larva to move through the instar phases, depending on environmental factors such as salinity, temperature, and food quality and quantity. It survives mostly on the benthic algae Nitzshia frustrulum, but also consumes the blue-green algae Oscillatoria, a green algae named Ctenocladus circinnatus, and a few protozoa (Herbst, 1986).
The anatomy of the larva is suited well for Mono Lake. The alkali–fly possesses a structure known as a respiratory siphon that extends into two respiratory tubes. It is believed that these tubes are utilized for breathing at surface levels (Cash, 1994). The lime gland can be observed through the transparent body of the larva. This functions as a kidney, removing carbonate ions from the blood. This adaptation allows the alkali-fly larva to thrive amongst the high level of carbonates in Mono Lake. Inside of the lime gland the carbonate is mixed with calcium, forming limestone. Limestone is stored within the body of the larvae, to be used as its source of calcium for its diet. Since Mono Lake has a low level of available calcium for the alkali–fly’s diet, it has adjusted accordingly (Simeone, 2001). As the insect advances through the larva stage, it seeks a hard substrate to cling to with its "Prolegs." The larva’s prolegs are claw–like appendages that allow attachment to predominantly solid substrates. Without prolegs, the larva could easily be dislodged and swept to the surface of the lake, where its life would end from predation (Herbst, 1990). Most often, the larva will chose geologic structures known as "tufa towers" to anchor to. Tufa are composed of a mineral called thinolite, also known as calcium carbonate or limestone. Tufa form when calcium brought to the lake in spring water combines with carbonates of Mono Lake (Hill, 2006). After the 25–30 day larval period, the insect’s skin hardens and it enters an immobile, non–feeding pupate stage on the Tufa (Herbst, 1986). This transformative stage lasts one to three weeks, fluctuating with water temperature. As it exits the pupa stage, the limestone stored in the lime gland is excreted, adding a biological component to the tufa tower.
Agreements – Helpful or Ignored?
After the LADWP began pumping groundwater in 1970, and the effects were clearly evident to residents in the area, Inyo County filed suit under the California Environmental Quality Act. Nineteen years of litigation ensued, ending with the signing of the Inyo–LA Long Term Water Agreement (LTWA). This requires that pumping be properly managed to keep a water supply that is sufficient for both the Valley ecosystems and the city of Los Angeles water demand. According to the OVC’s website, the LTWA has not been as effective as hoped.
Then in 1997 the Memorandum of Understanding between several actors including the City of Los Angeles, Inyo County, and the Owens Valley Committee, was signed. Its intent was, according to the OVC website, to increase monitoring of vegetation that is dependent on groundwater in the Valley, revegetation in areas severely damaged by the LADWP, recovery of areas damaged by drought, rewatering of a 60–mile stretch of the Owens Rover originally diverted in 1913, and monitoring of natural resources on LADWP land. Again, the Owens Valley Committee finds the LADWP not sufficient, claiming that they are still pumping harmful amounts of groundwater making revegetation, rewatering, and overall recovery difficult.

Exposed Tufa Towers, image photographed by Brett Leigh Dicks
The adult alkali–fly exits the case. The new adult fly may walk for up to fifteen minutes in the water prior to its float to the surface via an encasing air bubble (Foley and White, 1989). When the alkali–fly climbs to the surface, its wings dry. The rest of its life will be spent feeding and breeding on the shore of the Mono Lake.
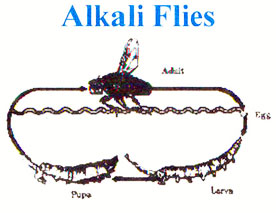
Life cycle of the alkali fly. Source: Mono Basin Virtual Visitor Center.
Food is necessary for successful reproduction. When the fly is ashore, it grazes on algal and detrital materials. The alkali–fly also has the option of diving to the high quality benthic algal mats underneath the water. Mono’s flies also take advantage of this ability during egg–laying. No particular observable behaviors are used to choose partners and it is thought to be random selection (Herbst, 1986). Female flies produce an average of ten eggs per day. This continues over the course of the two weeks. Each day, the female dives to the benthic mats beneath the surface of Mono Lake to deposit the eggs and begin the lives of her offspring. A typical lifespan of the alkali–fly is two weeks (Simeone, 2001). However, many do not make it to this age because the fly is one of two major sources of food for millions of larger predators in the Mono Basin.
Planktonic Algae
Planktonic Algae are the other major primary producer in the Mono Lake ecosystem, alongside the benthic algae. Unlike benthic algae, planktonic algae are unicellular. While the ideal temperature conditions for growth remain the same as for benthic, between one and six degrees Celsius, the location under water differs. Planktonic algae are more likely to be found in higher concentrations in the upper portions of Mono Lake’s depths. Planktonic algae will not cluster into mats on the bottom, as seen with the benthic algae population. Instead, they float in the upper layers, moving slightly with two weak flagella. The planktonic algae have one major predator in Mono Lake.
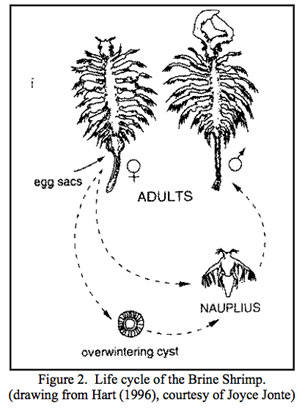
Brine Shrimp
Trillions of brine shrimp flourish in the waters of Mono Lake, grazing on planktonic algae. An estimated four to six trillion Artemia monica brine shrimp live in the lake in the warmer summer months (Mono Lake Committee Website). This species is unique in that it is only found in Mono Lake. In the springtime, as the water’s temperature increases, the cysts and immature shrimp begin to develop. After rising from the mud at the bottom of Mono Lake, the shrimp spend eight to twelve weeks reaching full maturity. Over this time period, Artemia monica undergoes fourteen molts. Upon maturation, the females give live birth to their offspring after copulation. The second–generation shrimp will procreate in a different manner. Instead of live birth, the female will lay a cyst that drops to the bottom of the lake. The cycle begins once more as the cyst develops through the next spring (GORP Website). The brine shrimp lives a life of minimal competition because the main consumer other than the shrimp is the alkali–fly, which targets mostly benthic algae. However, both the alkali–fly and the brine shrimp often fall to the same predators.
Throughout the twentieth century, LA built the aqueduct, damming streams and creeks, creating reservoirs, and pumping groundwater. This resulted in a series of lawsuits and agreements between the City of Los Angeles and several players on the Owens Valley side such as the Owens Valley Committee. Groundwater pumping became especially destructive to plant and animal life in the Valley between 1970 and 1990, resulting in the Memorandum of Understanding and the Lower Owens River Project. Unfortunately, the OVC has found the City of Los Angeles’s effort to restore life and vegetation in the lower parts of the valley insufficient. To fix this problem, the Committee urges interested individuals to get involved and offer alternative plans or ideas to monitor the LADWP’s involvement in and upkeep of the Valley.
Groundwater has proven to be a hot topic. LA not only diverted surface water from streams, creeks and tributaries in the Valley for years, but also in the 1970s they began pumping groundwater, which is not easy to replenish. Coupled with the worldwide increase in temperature, pumping groundwater is making the depleting groundwater resources dissolve quicker, which in turn affects the vegetation and wildlife in the area. Various plant species, birds, and land mammals live in the area, living off of groundwater supplies. The combination of the LADWP pumping groundwater and the natural decreasing amount of snowmelt and runoff has had dyer effects on the vegetation and wildlife, drying out the valley to an extremely harmful level.
Waterfowl
The Mono Basin supports over eighty species of waterfowl and birds. Though all of these species do not consume brine shrimp or alkali-flies, the birds of greatest populations do. Mono Lake houses approximately 40,000 to 65,000 Larus californicus, or California Gulls every April. This is the second largest California Gull colony in the world. In the spring, the gulls seek Mono Lake as a sanctuary to raise young. On islands of the lake (primarily Negit Island), thousands of California Gulls choose a region and begin scratching into the ground. With an impression decorated with feathers and various other materials, the female gull may begin egg–laying. A California Gull lays up to three khaki–colored, black speckled eggs. In June, the eggs will hatch and downy California Gull chicks are born. Adult California Gulls continue to enjoy a buffet alkali–flies and brine shrimp as the chicks fledge in July. In early August, the chicks are nearly full grown. The departure of parent and offspring California Gulls begins. The birds are off to their wintering grounds, returning to Mono Lake again the following April (Mono Lake Committee Website).
Image Courtesy of Andrew Ford
Other key birds to the Mono Lake area include Eared Grebes, Snowy Plover, and Wilson’s and Red–Necked Phalaropes. Thirty percent of North America’s Eared Grebe population stops at Mono Lake during migration. Within recent years, over two million Grebes have been spotted on the Mono Lake Grounds (Audubon Website). This is a crucial environment for the survival of the Grebe. Mono Lake also houses eleven percent of the Snowy Plover population (GORP Website). The approximately 20,000 Phalaropes that visit Mono Lake complete their molt before continuing the journey to South America (Audubon Website).
While the birds discussed above are species of highest concentrations in the area, there are many other mentionable waterfowl or birds. Mallards, Northern Pintails, Northern Shovelers, Canadian Geese, Ruddy Ducks, Gadwalls, Green-winged Teals, and Cinnamon Teals are commonly identified around Mono Lake. Occasionally, an encounter with a Lesser Scaup, Bufflehead, Snow Goose, or a migrating Tundra Swan is possible (Mono Lake Committee Website). The birds have a feast at Mono Lake, but they also have a few predators to watch out for. Predators of waterfowl at Mono Lake include coyotes, raccoons, bobcats, Great–Horned Owls, Golden Eagles, and hawks (Mono Lake Case Study).
Humans
Humans could be given credit as the final group of organisms with a role in the Mono Lake ecosystem. Over the ages, the influence upon the ecosystem has changed with the ideals of the area. Long before the arrival of European settlers, a Native American population known as the Kuzedika lived in the Mono area. They were known to travel throughout the area, gathering various foods. The name Kuzedika is roughly translated to "fly eater." The reason being that it was common for this group to spend time at Mono Lake during the pupa stage of the alkali–fly. Each summer, the Native Americans would wade into the shallow waters of Mono with weaved tools. They would use the tools to collect many pupa of the alkali–fly. The pupa would be dried and the outer layer of skin flaked off. The Kuzedika would eat them as a sort of cereal or use them for trade. The high nutritional value of the alkali–fly pupa made it a valuable commodity. Around two hundred Kuzedikas lived in harmony with Mono Lake year round, causing minimal disturbances in the ecosystem.
The next wave of human influence came with the miners of the area in the 1860’s. The nearby town of Bodie was packed with those looking to score a small fortune in the gold rush. In the 1880’s, a railway was built to transport lumber into the town for building, heating, and cooking. The mining town didn’t seem to have any direct effects on Mono Lake. However, the waste sediment from the mining process, loose sediments from tree harvesting, and river pollution surely took a toll on the wildlife.
In 1941, diversions of massive amounts of water from the rivers near Mono Lake for the people and businesses of Los Angeles began. As a result, the volume of the lake was cut in half and the salinity doubled. Scientists feared the extinction of species that inhabited the lake. With only brine shrimp and alkali–flies supporting the millions of migrating birds every year, the decline of one species could be devastating. By 1995, the lake had dropped forty feet from the pre–diversion level. Nesting birds were in great danger. A land bridge to islands such as Negit would spell disaster for the ecology of Mono Lake. Predators could easily reach the nests of birds and destroy the sanctuary. Researchers raced to provide evidence for the necessary increased water input. Alongside researchers, the Mono Lake Committee worked to improve diversion policy with Los Angeles. Over time and trials, L.A. would finally slow its diversions. The immediate increase of flow had a negative acute impact on the wildlife. However, now the lake has since risen to a much healthier level and maintains a positive population of extremophiles, alkali–flies, brine shrimp, and migratory birds. It looks as if Mono Lake will have a positive future and the ecosystem will remain in tact and more sensibly cared for in years to come (Mono Lake Committee Website).
Conclusion
With a better understanding of each organism’s role in the ecosystem of Mono Lake, humans learn the lake’s true nature. With this knowledge, the ecological role of the human in the Mono Lake community can be one of low negative impact on all of the species of organisms that inhabit the ecosystem. As research continues, the optimum conditions for life in Mono Lake can be found. By applying scientific research to policy and practice, humans can improve the conditions of Mono Lake and ensure that future generations will have the opportunity to visit this astonishing portion of the earth.Works Cited
Audubon: Birds and Science [-118.984, 37.9969] – Mono Lake Basin. Audubon: Birds and Science [-118.984, 37.9969] – Mono Lake Basin. National Audubon Society, Inc., 2012. Web. 9 June 2012. website.
Cash, Clark; Bradley, T.J. 1994. External morphology of the alkali fly (Ephydra hians) Say at Mono Lake, California (USA) in relation to physical habitat. Journal of Morphology 219(3), March: 309–318.
Dicks, Brett Leigh. Tufa Towers. N.d. Photograph. Micro*scope. Mar. 2006. Web. 8 June 2012. website
Foley, C.; White, B. Occurrence of Ephydra hians Say (Diptera: Ephydridae) in deep water in Mono Lake, California. Bulletin Southern California Academy of Sciences 88(1) (1989): 40–41.
Herbst, D.B. 1990b. See Superior Court for the State of California for the County of El Dorado. 1990.
Herbst, David B., and Timothy J. Bradley. Salinity And Nutrient Limitations On Growth Of Benthic Algae From Two Alkaline Salt Lakes Of The Western Great Basin (Usa)1. Journal of Phycology 25.4 (1989): 673-78.
Herbst, D. B. Comparative studies of the population ecology and life history patterns of an alkaline salt lake insect:Ephydra (Hydropyrus) hians Say (Diptera: Ephydridae). Ph.D. thesis, Oregon State University, Corvallis. (1986):206 pp.
Herbst, D.B. Distribution and abundance of the alkali fly at Mono Lake, California in relation to physical habitat. Hydrobiologia 197 (1990): 193–205.
Hill, Mary. Geology of the Sierra Nevada. Berkeley: University of California, 2006.
Humayoun, S. B., N. Bano, and J. T. Hollibaugh. Depth Distribution of Microbial Diversity in Mono Lake, a Meromictic Soda Lake in California. Applied and Environmental Microbiology 69.2 (2003): 1030–042.
Inyo National Forest. Great Outdoor Recreation Pages (GORP), 2012. Web. 9 June 2012. website.
Jiang, S., G. Steward, R. Jellison, W. Chu, and S. Choi. Abundance, Distribution, and Diversity of Viruses in Alkaline, Hypersaline Mono Lake, California. Microbial Ecology 47.1 (2004): 9–17.
Lawrence, Steve. Naturalists Trying to save the 'Dead Sea of the West. The Modesto Bee 18 Aug. 1985: B–4. Google News. Web. 10 June 2012. website
MacIntyre, Sally, Kevin M. Flynn, Robert Jellison, and José Romero. Boundary Mixing and Nutrient Fluxes in Mono Lake, California. Limnology and Oceanography 44.3 (1999): 512–29.
Madrid, V. M., G. T. Taylor, M. I. Scranton, and A. Y. Chistoserdov. Phylogenetic Diversity of Bacterial and Archaeal Communities in the Anoxic Zone of the Cariaco Basin. Applied and Environmental Microbiology 67.4 (2001): 1663–674.
Mono Lake Case Study. TED Case Studies. Web. 10 June 2012. website
Mono Lake Committee. 2012. Web. 9 June 2012. website
Oremland, R. S., S. E. Hoeft, J. M. Santini, N. Bano, R. A. Hollibaugh, and J. T. Hollibaugh. Anaerobic Oxidation of Arsenite in Mono Lake Water and by a Facultative, Arsenite–Oxidizing Chemoautotroph, Strain MLHE–1. Applied and Environmental Microbiology 68.10 (2002): 4795–802.
Simeone, Mono. The Biogeography of Mono Lake Alkali Fly (Ephydra Hians). San Francisco State University Department of Geography. N.p., Dec. 2001. Web. 10 June 2012. website