Geology and Natural Heritage of the Long Valley Caldera
Hydrothermal Alteration
Marcella Yant
Abstract
Changes in rock condition, a heat source, and pathways for material to travel through all must be present for hydrothermal alteration to take place. Hydrothermal alteration is the interacting of hydrous liquids, called hydrothermal fluids, reacting with surrounding rock. During the reaction, the composition of the rock is changed, through the replacing of minerals. This process creates beautiful scenery and can also be harvested for ore used economically.
Introduction
In the Sierra Nevada, and numerous other locations, one can observe rocks that have experienced a strange phenomenon. The rocks have color intertwining through them, resulting in a
mosaic of beauty. These colors are caused by hot liquids, called hydrothermal fluids, which cause a composition change in the minerals. Alterations can be commonly viewed with chlorite, quartz, and
serpentine. This process is known as hydrothermal alteration.Three changes in conditions can cause an alteration in a rock; change in temperature, pressure, or chemicals. A shift in these conditions can result in a mineral deposit or an ore deposit. Ore deposits like lead, (the mineral galena), zinc (the mineral sphalerite), and copper (the mineral malachite) can be created by hydrothermal alteration. The process, by which this occurs, first starts with a heat source to raise the temperature of water. [Encyclopædia Britannica Online] There are few sources, for both of the resources listed. Magmatic rocks can exsolve water when they reach a certain pressure where water is no longer soluble in the rock. The water is given off in the rock’s last phases of cooling. The next source is water created by dehydration reaction in metamorphic rocks. When the temperature increases the hydrous minerals that normally exist at a lower temperature recrystallize their structure into anhydrous minerals. The anhydrous minerals can exist in a higher temperature environment. When the hydrous minerals make the conversion to anhydrous minerals water is given off. The water may have bound to some metal ions, taking the metal with it as it exits the mineral and goes into the surrounding rock. The water and metals can seep into the cracks in the rock and precipitate to form ore deposits or minerals. [http://www.dmtcalaska.org/course_dev/explogeo/class08/notes08.html] The last source is groundwater that could be heated by magma. Other sources could exist. [Craig et al., 1988]
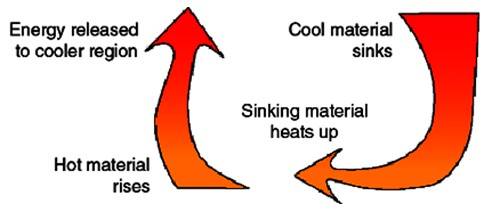
Hydrothermal fluids that are in a plutonic environment, an environment with molten material that has cooled below the surface of the earth, follow a convection pathway. The hottest water is right above the source of heat and rises rapidly, vertically and then spreads out horizontally and then back down again, vertically, as depicted in both Diagram 1 and 2. Diagram 1 is a very basic depiction of how the density as a function of temperature alters the buoyancy of a substance, in this case water. As water gains energy from a heat source it expands and rises up. Since heat flows to from areas of high concentration to those of lower, the heat is given off as the environment around the water is cooler. This occurs at shallower depths rather than deep because of the temperature of the country rock. As the heat is given off the water loses its buoyancy and retreats back down to be heated up again, creating a convection path. The convection path of the hydrothermal fluids, as stated above, can be best described with the analogy of heating water in a cooking pot, Diagram 2. The water rises above the flame and reaches the surface, where the atmosphere is cooler, and gives off heat. The cooler water then circulates down the sides of the pot to be reheated.
Diagram 1: Simplified Hydrothermal Fluid Convection Path; Figure reproduced from North Arizona University Meteorology [2009] (exact reproduction)
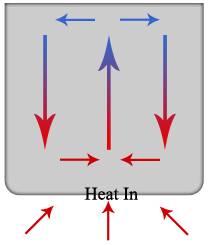
Diagram 2: Water Convection Path in a Cooking Pot; Figure reproduced from Wikimedia [2006] (exact reproduction)This path stems from a normally deep igneous intrusion. There are rocks that allow water to pass with little resistance, these are permeable host rocks. A host rock is the rock surrounding an ore deposit. An Example of a permeable host rock is coarse-grained sandstone. There are also impermeable host rocks, like shale. The impermeable host rocks can act as a dam which can lead to a saturated area of hydrothermal fluid, resulting in a large concentration of minerals in the dammed area. [Craig et al., 1988]
The presence of faults and fractures provide a great environment for hydrothermal alteration to occur. A well-developed fault system makes a great host rock because mineralization can take place in the large cracks, creating grand veins. When fault occurs breccia and gouge can be formed which is a great subject for replacement mineralization. Breccia is rock consisting of jagged pieces implanted in a fine-grained medium. It is formed by the altering of metamorphic rock and held together by carbonate rich cement. Gouges are poorly consolidated rocks that have a clay-rich matrix and contain porphyroclasts that make up less than half of the mass. The veins created in faults and fractures range from large to fine-grained and can have a vuggy texture. Vuggy refers to a mineral layer that is composed differently from its surrounding rock. Another reason fault zones are suitable for hydrothermal alteration is because of their great circulation of water. [http://www.dmtcalaska.org/course_dev/explogeo/class08/notes08.html]Patterns that are frequently found around ore deposits are referred to as hydrothermal alteration zoning. The patterns are caused fluctuations in temperature, fluid chemistry, or gas content. For example, if the temperature decreases then minerals can be altered from high temperature minerals to low temperature minerals. Zoning can form varying patterns including concentric shell, linear, irregular, and complex. [http://www.dmtcalaska.org/course_dev/explogeo/class08/notes08.html] For example, a porphyry copper deposit has a concentric shell. It has a core of potassic alteration which forms potassium feldspar (KAlSi3O8) and biotite (K(Mg, Fe)3AlSi3O10(F, OH)2). Both of these minerals are commonly found in granite. The middle zone of the porphyry copper deposit is phyllic containing a complex of quartz (SiO2), sericite, and pyrite (FeS2). The outer zone of the porphyry copper deposit contains a quartz (SiO2), chlorite ((Mg,Fe)3(Si,Al)4O10(OH)2·(Mg,Fe)3(OH)6), and carbonate (CO32-) complex and may also contain epidote (Ca2Al3(SiO4)(Si2O7)O(OH)), albite ((Na,Ca)(AlSi3O8)), or adularia (KAlSi3O8). [Mineral Information Institute, 2008].
There are two general categories of minerals, high temperature and low temperature, which are broken into sub categories called alteration types. The most common types are propylitic, sericitic, potassic, albitic, Silification, Silication, carbonatization, alunitic, argillic, zeolitic, serpentinization and talc alteration, and oxidation. [http://www.dmtcalaska.org/course_dev/explogeo/class08/notes08.html]
Propylitic alteration results in the formation of the minerals chlorite, epidote, and actinolite. [Barnes, 1979] These minerals cause the rock’s color to be changed to green during alteration. The minerals are created from the decomposing of iron and magnesium containing minerals. The minerals that are generally replaced are biotite, amphibole, pyroxene, and sometimes feldspar. For this reaction to take place a low temperature is required. [http://www.dmtcalaska.org/course_dev/explogeo/class08/notes08.html]
Sericitic alteration results in sericite, a fine-grained mica, replacing potassium-feldspar and plagioclase during the process. [Barnes, 1979] The presence of sericite can be noted by its characteristics of slick texture, easily scratched surface, and can be white, yellow, golden brown, or green in color. Sericitic alteration occurs in an acidic, low pH, environment. Phyllic alteration includes sericite and quartz and may be connected with sericitc alteration when the event is associated with porphyry copper deposits, when pyrite is formed. [http://www.dmtcalaska.org/course_dev/explogeo/class08/notes08.html].
Potassic alteration involves the minerals biotite, potassium feldspar, and adularia formed in place of plagioclase and mafic minerals. [Barnes, 1979]. Potassium enrichment occurs at a high temperature. This type of alteration can take place prior to all of the magma has crystallized, forming flowing, irregular veins. This alteration can take place in plutonic environments at a considerable depth, creating orthoclase. It can also take place in superficial, volcanic environments and adularia is created. [http://www.dmtcalaska.org/course_dev/explogeo/class08/notes08.html]
Albitic alterations result in the formation of albite, also known as sodic plagioclase. Normally signifies a fortification of sodium and sometimes creates white mica paragonite that is rich in sodium. Albitic alterations are a high temperature process. [http://www.dmtcalaska.org/course_dev/explogeo/class08/notes08.html]
Silification involves adding secondary silica to a complex. Diagram 3 shows a chart of the primary silicate mineral and its alteration product. Volcanic ash is altered into vermiculite and montmorillonite. Olivine is altered into limonite, serpentine, and talc. Pyroxenes and amphiboles are altered into vermiculite, montmorillonite, limonite, serpentine, chlorite groups, and talc. Plagioclase feldspar and potassium feldspar are altered into kaolinite, bauxite, and illite. Muscovite mica is altered into illite. Also given on the chart is the resistance of the minerals, volcanic ash being the least and muscovite being the most resistant. Silification has different styles, including silica flooding and stock works. Silica flooding is when rock is replaced with chalcedony, microcrystalline quartz. Stock works are when close-spaced fractures are filled with quartz forming a network. Silification oc
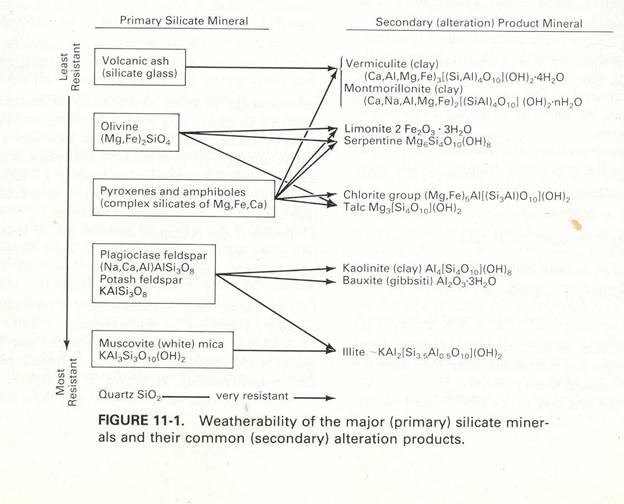
Diagram 3: Silicate minerals resistant and product chart; Figure reproduced from Craig [1988] (exact reproduction)Silication involves silicate minerals with or without quartz. The process involves creating a silicate mineral to insert silica to a complex. Minerals commonly included in this event are biotite, garnet, and tourmaline and are formed at a range of temperatures. A skarn can be formed when silicate minerals assemble in the presence of igneous intrusions with carbonate sedimentary rocks. Greisenization can also occur. Greisen is a rock with parallel veins of quartz, muscovite, and other minerals. The parallel veins can be found in the roof zone of the surrounding rock or pluton if open fractures are present. [http://www.dmtcalaska.org/course_dev/explogeo/class08/notes08.html]
Carbonatization involves the formation of carbonate minerals like calcite, ankerite, and dolomite. This process may also involve the addition of talc, chlorite, sericite, and albite. This event often produces a pattern around its deposits, normally with iron-rich types close to the deposit. [http://www.dmtcalaska.org/course_dev/explogeo/class08/notes08.html]
Alunitic deals with the formation of alunite, a potassium aluminum sulfate mineral. It is a type of alteration involved with hot springs. Alunite indicates a possible high concentration of SO4 gas that is formed from oxidizing sulfide minerals. [http://www.dmtcalaska.org/course_dev/explogeo/class08/notes08.html]
Argillic alteration is the formation of clay minerals including kaolinite, sinectite, and illite generally replacing plagioclase. [Barnes, 1979] Generally the event takes place at a low temperature and can occur in atmospheric conditions. The bleaching out of feldspars signals an argillic alteration has occurred. There is a subcategory of this type of alteration called advanced argillic. It involves kaolinite, quartz, hematite, and limonite which exist in a low pH, very acidic environment. When the temperature rises to high rather than low, pyrophyllite replaces kaolinite in the process. [http://www.dmtcalaska.org/course_dev/explogeo/class08/notes08.html] Bentonite, a clay mineral made up of smecite minerals (saponite, montomorillonite, and hectorite), can form from volcanic ash that has undergone hydrothermal alteration. This occurrence normally is from volcanic ash that has been deposited distally from its source or eruption. The location of the ash is normally into a body of water that breaks it down into the clay minerals that form bentonite. The volcanic source is normally rhyolitic or andesitic, meaning high or intermediate in silica content. In California hectorite deposits may have resulted from the hydrothermal alteration of tuff, sourcing of the hydrothermal fluid being from hot springs in alkaline lakes. [Craig et al., 1988]
Zeolitic alteration deals with the formation of zeolite minerals generally in volcanic environments, but can be located distally. The zeolite in this environment replaces the glass matrix. This process occurs at a low temperature so it has to take place in the stage before a volcanic eruption or activity and at shallow depths. [http://www.dmtcalaska.org/course_dev/explogeo/class08/notes08.html]
Serpentization and talc alteration involve, in order, the minerals serpentine and talc. When serpentine is formed it is characterized by its waxy appearance, greenish coloring, and soft surface. The host rocks involved are mafic to ultramafic, which have a higher iron and magnesium concentration. Serpentization takes place at a low temperature. Talc resembles serpentine but is paler. Talc signifies a larger amount of magnesium when being crystallized. [http://www.dmtcalaska.org/course_dev/explogeo/class08/notes08.html]
Oxidation often replaces sulfide minerals with iron oxides because sulfide minerals are easily worn down. Oxides form best in shallow places because of the availability of oxygen. Oxidation can take place in low to moderate temperatures and in atmospheric and surface conditions. [http://www.dmtcalaska.org/course_dev/explogeo/class08/notes08.html] Iron, manganese, sulfur, carbon, and hydrogen are the elements that react in oxidation-reduction processes that occur during hydrothermal alteration. The composition of the fluids oxidation can be determined by observing the valence state of the above elements after the mineral has been created. [Barnes, 1979]
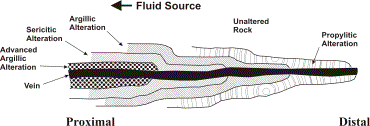
Zoned vein deposits are found by the side of fractures and faults and are recognized by a replacement occurrence. These deposits tend to be composed of a variety of metals, thus making them polymetallic. The deposits are mined for copper, lead, zinc, and silver. The zoned deposit can be broken down into two categories: veins with porphyry base metal deposits and those without. The veins in the first category tend to form at a lower temperature and at a later phase of mineralization. However, the temperature is variable; there are higher temperature minerals closer to the pluton and lower temperature minerals farther from the pluton. The high temperature minerals are rich in copper and have sulfide minerals which a high metal to sulfur ratio. The low temperature minerals are rich in lead and zinc and have sulfide minerals but have a low metal to sulfur ratio. Butte, Montana is a suitable example for zoned vein deposits. The veins have a core of porphyry copper-molybdenum, as shown in Diagram 4, below. Closer parts of the vein have undergone advanced argillic alteration and extend to sericitic alteration in the next zone outwards. Parts farther from the vein have recognizable propylitic alteration. The veins in the second category exist at a more central, consistent temperature over a wide region. Zoning that takes place in the veins without porphyry base metals alter as a function of fugacity in sulfur. [http://www.dmtcalaska.org/course_dev/explogeo/class08/notes08.html]
Diagram 4: Zone Vein Deposit; Category 1; Figure reproduced http://www.dmtcalaska.org/course_dev/explogeo/class08/notes08.html (exact reproduction)
Typical zoning that occurs in locations stemming the earth have the ideal sequence of the following, (starting from the core extending outwards): molybdenite, arsenopyrite, pyrrhotite, pentlandite, stannite, chalcopytrite, sphalerite, tetrahedrite, galena, acanthite, gold, tellurides, stibnite, and cinnabar. This sequence is not perfectly exhibited in any deposit. [Barnes, 1979]
Hydrothermal solutions are responsible for the formation of most of the ore deposits found on this earth. Water for these fluids come from two sources of water, rainwater and seawater, and are heated by two main methods. At depths of three thousand feet, deeper rock is hot and boosts water in its ability to dissolve solutes. Thus the water becomes mineral containing by dissolving sodium chloride, magnesium chloride, calcium sulfate, silicon dioxide, and trace amounts of scarce metals. As the metal carrying solution fills in cracks, accompanied by a decrease in pressure or a decrease in temperature, minerals begin to precipitate out. The second source of water is formed from the cooling of magma. When magma is made from the melting of rock in the lower crust and upper mantle, water is combined into the liquefied mixture. Granites and diorites contain a high water percentage from forming the crust, where water is obtained more readily. During cooling and crystallization water is exsolved along with some minerals forming a hydrothermal solution. Fluid flow is then created by a heat source like magma and the fluid expands and rises because of its increase in buoyancy. An example a hydrothermal system, like that mentioned above, are the hot springs at Yellowstone National Park. Rainwater accumulated and was heated by nearby magma. As a hydrothermal solution rises it can cool, indicating a fluid with a pH slightly on the low side, and it reacts with rocks like limestone. As the pressure drops some gases are no longer soluble in water and exsolution and/or boiling takes place. If the point of saturation is met, minerals will be precipitated. The compositions of the hydrothermal solution changes as minerals are removed, which can result in a zonal pattern. [Craig et al., 1988]
A respectable example of a hydrothermal system, as observed by Richard David Harvey, is the Goldfield District in Nevada. Harvey used analysis of microscope and x-ray information to prove the existence of a pattern in the alteration. In the location observed, three prominent zones of alteration were determined. The core zone being that of quarts and alunite. The next zone outward consists of an argillic zone that outlines the core zone. The outermost zone is the propylitic zone. [Harvey, 1960]
The quartz-allunite zone is mainly composed of alunite, but also contains quartz, kaolinite, jarosite, pyrite, hematite, halloysite, gypsum, epidote, rutile, and traces of ore. There is a distinct cutoff between this zone and the argillic zone. The rocks in this zone form parallel veins because of their resistance. The rocks are also characterized by small grains, making the rock very smooth. Outcrops are frequently found with layers that differ in minerals from the rest of the piece (vuggy) or larger minerals that are imbedded in a fine-grained like cement (breccia), and are angular in shape. [Harvey, 1960] The host rock is originally porphyritic in texture, meaning it is a combination of coarse and fine-grained. Porphyritic textures normally result from magma cooling slowly underground (allowing time for crystal growth), and then being expelled and cooled quickly above the surface (resulting in no-fine-grained crystals).The argillic zone is the habitat of minerals like montmorillonite, illite, and kaolinite. Veins separate the argillic zone from the propylitic zone. Within this zone, two subzones exist, a zone of montmorillonite surrounding a core subzone of illite and kaolinite. The wideness of the argillic zone is not constant and can overlap which results in the lacking of a propylitic zone. Normally this zone is symmetrical along veins. In the montmorillonite subzone the mineral makes up ten to twenty percent of the rocks. As the zone begins to come upon the quartz alunite zone, montmorillonite dies out slowly. Within the montmorillonite subzone alterations of plagioclase is found and sometimes altered to illite. Biotite is also found in this subzone and altered to illite and iron and titanium create ilmenite and leucoxene. The montmorillonite has traces of kaolinite, hematite, ilmenite, and magnetite found within it. The kaolinite is detected through the use of x-rays because of its fine grains. In the illite and kaolinite subzone, the minerals take up around ninety percent of the rock. In this subzone, biotite is altered into illite mostly with some occurrences resulting in kaolinite. [Harvey, 1960]
The propylitic zone has the smallest amount of alteration out of the three zones. Its occurrence results from the immediacy of the other zones. During alteration, biotite is changed to chlorite, epidote, and mica. Plagioclases, like augite and pigeonite that can be found in the andesite and dacite have some amounts of alteration into calcite and chlorite, but not much. Unchanged feldspar is also found in this zone and can contain traces of calcite. Rocks altered in the propylitic zone show variety from fresh rock where mafic phenocrysts have been colored brown through alteration instead of dark green. Texture of these phenocrysts does not change. Rock from this zone exsolved gases, when treated with hydrochloric acid, and leaves calcite behind. [Harvey, 1960]
From the stratification and composition of the Esmeralda lake sediments the pressure during alteration can be determined. The Esmeralda lake sediments superimpose over the dacite, andesite, or latite. The samples used are found in mineralized chunks in the sediment and have lateral patterns. The veins that went through silification were created and eroded partly, before the lake was formed. Rocks that go through alteration at the surface have a pressure of one atmosphere. They are considered the lower limiting pressure. The upper limiting pressure can be found by using the rocks that are pushing down on the location in which the deepest mineralization is occurring. Cambrian shale coming in contact with latite flow at a depth of one thousand feet is the deepest alteration has been found. The equation used to determine pressure is given below:
Equation 1
In this equation P is pressure, D is the density of overlying rocks, h is the depth (measured in feet), and the constant is gravitational acceleration and conversions to feet. The density of the dacite is 2.63 g/cm3 and can be plugged into the equation, as done by Harvey, at depths of 200 feet, 300 feet, and 1000 feet. The pressures found in order of the preceding depths are, 15.5 atmospheres, 23.3 atmospheres, and 77.5 atmospheres. In the case of open fissures, hydrostatic pressure is used, which uses the overlying fluid weight instead of the overlying rock weight. The density of the fluid is estimated at one and plugged into the equation, again at the depths of 200 feet, 300 feet, and 1000 feet. The pressures found in order of the preceding depths are, 5.90 atmospheres, 8.85 atmospheres, and 29.5 atmospheres. The range between the two sets of data is influenced by the difference in density of the two sources of pressure. Looking at the minerals that exist in the hydrothermal environment allows one to harvest a temperature at which the process could have occurred. Since alunite and kaolinite both decompose at temperatures above 500° C. it is know that the process temperature was lower than this value. Also kaolinite does not form naturally if the temperature is higher than 400°C. Jarosite is not stable at temperatures above 345°C. Xonotlite undergoes decomposition if the temperature reaches above 300° C. Alunite forms at 200° C when at a pressure of 15 atmospheres. From the information given above it can be estimated that the alteration occurred between 200°C and 300°C. [Harvey, 1960]
In the parts of California visited on the 2009 Sierra Nevada Class some hydrothermal alteration could be seen. At Casa Diablo Geothermal Plant, evidence of hydrothermal alteration could be seen around the edges of the fumaroles. The fumaroles emit vapors of water and other gases, normally producing hydrogen sulfide. When hydrogen sulfide is altered through oxidation to sulphuric acid and native sulfur, hydrothermal alteration occurs. [USGS General Interest Publication, 1985] Casa Diablo is positioned on the southwest side of the resurgent dome [USGS Website, 2001]. Hot springs can also be associated with hydrothermal alteration, like Hot Creek. Hot creek is 25 miles south of mono lake. Hot Creek includes not only hot springs, but also fumaroles and geysers. The heat source for this system is the hot magma that can be found underlying this location, about 3 miles under the springs. [GORP, 2009] The Artists Palette was a lively combination of colors, created by hydrothermal deposits, and is located just north of Badwater Basin. It is caused by hydrothermal alterations in a volcanic deposit. As shown by Diagram 5 below, iron results in red, yellow, and pink, mica decomposition results in green, and manganese causes the purple.
Diagram 5: Artists Palette; Death Valley, California; Figure reproduced from Our Beautiful World at the Backroads [2008] (exact reproduction)
In order to have hydrothermal alteration there must be a change in the conditions of the rock, a heat source, and a pathway (like fractures) for material transport. Hydrothermal alteration occurs all over the world and can result in a number of beautiful landforms like the Artists Palette in Death Valley, California. Occurrences of hydrothermal alteration occur all over the world and are an important source for minerals. If veins are large enough they can be harvested and used economically. Geochemists can study the effects of the hydrothermal fluids and determine convection pathways through the earth, reoccurring patterns, structure of minerals, and crystallization. This can provide society with an outlook on scare minerals and metals. Hydrothermal alteration is an important process that provides the world with minerals, beauty, and studies in geochemistry.
Bibliography
Adams, David. "Hydrothermal Veins and Alteration." Introduction to Exploration Geology. Delta Mine Training Center. 3 Jun 2009 <http://www.dmtcalaska.org/course_dev/explogeo/class08/notes08.html>.
Barnes, Hubert. Geochemistry of Hydrothermal Ore Deposits. 2. New York: John Wiley and Sons, Inc., 1979.
"Casa Diablo Hot Springs and Geothermal Facility Long Valley, Caldera, California." USGS Website Nov 2001 Web.13 Jun 2009. <http://vulcan.wr.usgs.gov/Glossary/ThermalActivity/description_thermal_activity.html>.
"Common Minerals and Their Uses." Mineral Information Institute. 2008. Mineral Information Institute. 5 Jun 2009 <http://www.mii.org/commonminerals.php>.
"Convection." Wikimedia. 26 Sep 2006. Wikimedia. 16 Jun 2009 <http://images.google.com/imgres?imgurl=http://upload.wikimedia.org/wikipedia/commons/1/19/Convection.png&imgrefurl=http://commons.wikimedia.org/wiki/File:Convection.png&usg=__aEOEYGLtJyCLr50V2zTe-iZmnzo=&h=245&w=210&sz=10&hl=en&start=20&um=1&tbnid=4lE_B8RWiXqRaM:&tbnh=110&tbnw=94&prev=/images%3Fq%3Dconvection%2Bpot%26hl%3Den%26client%3Dfirefox-a%26channel%3Ds%26rls%3Dorg.mozilla:en-US:official%26sa%3DN%26um%3D1>.
Craig, James R, Brian J. Skinner, and David J. Vaughan. Resources of the Earth. Englewood Cliffs, New Jersey: Prentice Hall, Inc., 1988.
Harvey, Richard. Hydrothermal Alteration in the Goldfield District, Nevada (1960): 15-42. Print.
"Hot Creek Geologic Site." Inyo National Park. 2009. GORP. 13 Jun 2009 <http://gorp.away.com/gorp/resource/us_national_forest/ca/see_iny3.htm>.
"Hydrothermal mineral deposit." Encyclopædia Britannica. 2009. Encyclopædia Britannica Online. 17 Jun. 2009 <http://www.britannica.com/EBchecked/topic/279074/hydrothermal-mineral-deposit>.
Schmeig, Sebastian. "Death Valley National Park: Behind the extremes." Our Beautiful World at the Backroads. Jan 2008. World Press. 12 Jun 2009 <http://images.google.com/imgres?imgurl=http://betchaitluc.files.wordpress.com/2007/11/artist-palette.jpg&imgrefurl=http://betchaitluc.wordpress.com/2008/01/&usg=__091RHU4PTswDa2nvpevf6v037SM=&h=448&w=654&sz=82&hl=en&start=1&um=1&tbnid=hl4wndIXxDah4M:&tbnh=95&tbnw=138&prev=/images%3Fq%3Dartists%2Bpalette%2Bhydrothermal%26hl%3Den%26client%3Dfirefox-a%26channel%3Ds%26rls%3Dorg.mozilla:en-US:official%26hs%3D5Fx%26sa%3DG%26um%3D1>.
"Tilling." Volcanoes: USGS General Interest Publication 1985 Web.13 Jun 2009. <http://vulcan.wr.usgs.gov/Glossary/ThermalActivity/description_thermal_activity.html>.
Wittke, James. "Glossary Cc." North Arizona University Meteorology. 15 Feb 2009. Northern Arizona University. 16 Jun 2009 <http://www4.nau.edu/meteorite/Meteorite/Book-GlossaryC.html>.