Geology and Natural Heritage of the Long Valley Caldera
Anomalous Volcanic Gases
Jade Marks
ABSTRACT
This report provides a basic introduction to the concept of volcanic gases, particularly those which are not released during volcanic eruptions. These gases include carbon dioxide and sulfur based gases which escape from deep within the Earth's crust through fumaroles and gas seeps. This paper focuses on events and conditions at the carbon dioxide tree kill areas near Mammoth Mountain, California. A brief history is given, along with origin hypotheses, a description of potential hazards, and an outline of the precautionary measures that are now in effect. Along with a general description of potential hazards, this paper includes two examples of gas-related tragedies, one within the United Sates (at Mammoth Mountain), and one occurring overseas. Finally this paper seeks to inform readers of a few potential benefits that gas seeps, anomalies, and fumaroles may provide for mankind.
Sign marking a closed road where CO2 e missions have made the area unsuitable for tourist and recreational activity. Photo Credit: Jade Marks 2007
Brief Introduction to Volcanic Gases
One of the limiting factors in the explosiveness of a volcanic eruption is the concentration of gases contained within the magma body. Along with silica content, this factor determines the violence and in part, how dangerous an eruption will be for humans. Some major volcanic gases include, water vapor, carbon dioxide, sulfur dioxide, carbon monoxide, and hydrogen sulfide. Carbon dioxide and water vapor make up 90% of gas output, and while various other gases occur in relatively sparse amounts. Although they do not occur in vast quantities, these gasses can become erupted into the stratosphere and create acid rain (Volcanic Activity 216). While some gases are expelled when the volcano erupts, others, such as carbon dioxide and sulfur are known to make their way up to the surface through fissures and seepages during relatively inactive periods. Although the gases that erupt form a volcano are often the first type of gases that comes to mind, volcanic gases are being emitted at sites through gas seeps known as anomalies every day. This activity can occur over a period of years or even centuries prior to or after an explosion and often correlates with other volcanic activity such as earthquakes (Volcanic Activity 216). The study of these gasses is vital to scientists who wish to monitor volcanic activity and predict eruptions. Volcanic gases can even serve as a resource to mankind by providing clues to where the best hydrothermal energy sources are located. Not to be taken lightly, these seeping gases can also be quiet killers. Only knowledge and understanding can help mankind live in harmony with such exciting geological forces.
Gas Seeps and Anomalies
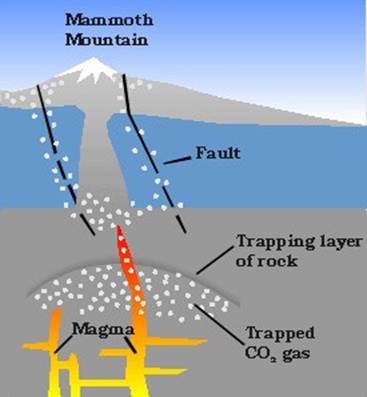
Diagram representing potential gas movement within the Earth's crust. ( Sorey 2000)
Gas seeps often occur in relation to volcanic activity. Magma, which is gaseous by nature, has been known to release its gases as it rises to the surface of the Earth. Because magma is less dense than the surrounding rock it is moved upward by gravity relative to the surrounding mantle and crust. As it rises, the pressure acting upon the body of magma changes, which allows for the release of otherwise dissolved gases (Farrar 1999). These gases have narrow margins through which they can travel, including fissures, lose sediment, and aquifers. Gases may follow the path of presently or previously existing ground water and reach the surface of the earth. When this occurs it can have damaging effects on the flora and fauna of the region. Often when gases can not reach the surface immediately they are stored in a chamber below the Earth's surface where they remain until a trigger is able to facilitate their movement (Farrar 1999).
Potential Hazards
Trees killed by carbon dioxide asphyxiation at Horseshoe Lake Tree-kill Zone. Photo Credit: Jade Marks 2007
There are various hazards associated with the presence of gas seeps. Abnormal concentrations of a given gas can be toxic to plants and animals, including unsuspecting people. Carbon dioxide, one of the most prevalent gases in volcanic gas seeps, may accumulate in low lying areas around snow banks or inside buildings. When this occurs, a human or animal wandering into the area can be in danger of asphyxiation from the toxic gas. This is one reason snow caves, tents, natural caverns, and other low lying structures are exceptionally dangerous in gas-hazard areas (Farrar 1999). Plants, which are unaccustomed to such altered soil composition, can suffer tremendously from exposure. Because plants were meant to absorb oxygen through their roots and carbon dioxide through their leaves, high levels of carbon dioxide in the soil can actually asphyxiate the trees (Sorey 2000). Carbon dioxide is a colorless gas that can not be detected by humans below concentrations of 10-20%. This means that extra caution must be employed when dealing with this particular hazard (Sorey 2000).
Mammoth Mountain , Long Valley Caldera
View of Horseshoe Lake Tree-kill zone from the top of Mammoth Mountain. (Sorey 2000)
The gas seeps at Mammoth Mountain , in the Long Valley Caldera of California are a fascinating example of this phenomenon of volcanic activity. Following a swarm of small earthquakes in 1989 the Forest Service noticed regions of dead and dying trees around the mountain. After drought and insect infestations were eliminated as causes, scientists discovered that the roots of the trees were being killed by exceptionally high concentrations of carbon dioxide gas in the soil (Sorey 2000, 1). While trees do need carbon dioxide for photosynthesis, it can only be used when absorbed directly by the leaves. The roots of a plant are meant to absorb other nutrients such as oxygen and minerals from the soil. Carbon dioxide coming in through the root system of a plant denies it of the oxygen needed for nutrient absorption. In these tree kill areas, the carbon dioxide makes up 20-95% of the gas content of the soil, resulting in a tree kill area that presently effects more than 100 acres (Sorely 2000). Normally gas concentrations consist of only 1% carbon and 30% concentrations can be deadly, which makes Mammoth Mountain a great concern for geologists (Sorey 2000). The oldest trees located in Mammoth Mountain tree kill area are approximately 250 years old, which leads geologists to believe that this age correlates with the most recent carbon dioxide seepage. Emission rates total close to 300 tons of CO 2 per day. Detrochronology, the study of dating using tree rings, shows a peak in gas emissions to have occurred in 1991which declined thereafter, stabilizing around 1996 (Sorey 2000).
Because gas and oil deposits often occur naturally throughout the Earth's crust, it is important to distinguish whether a given gas seep is a result of reservoirs, biotic activity, decomposition of carbon based rocks, or volcanic activity. In regard to Mammoth Mountain, the estimated output of 530 Mg/d is much larger than can be expected from biotic activity, notes one USGS report, and isotopic analysis of carbon and helium in the soil gas show that the source is deep seated (Farrar 1999, 11). Seismic data shows that the source material causing the CO 2 seepage is located from 2-18km below the surface. Most of the CO 2 seeps, known as anomalies, are on or near mapped fault lines. This is further evidence of a magmatic source. One hypothesis for the transport of carbon dioxide in the region is the existence of an extensive network of groundwater flows beneath Mammoth Mountain . According to the USGS, ground water occurs in a number of shallow perched aquifers and in one or more deeper flow systems in fractured rocks. The existence of a number of carbonated springs in the region support this theory. This also explains the lack of other volcanic gases in the area, because sulfur gases are less soluble than CO 2 (Farrar 1999, 11). The amount and rate of magma intruding into the shallow crust are two important factors in the onset of eruptive activity, and thus can greatly affect emission anomalies. Changes in the rate of CO 2 emissions may occur in response to new intrusions, therefore it is important for geologists to monitor these sites.
Regional Tragedies
A fumarole located at Mammoth Pacific Geothermal Power Plant G-2/G-3. Photo Credit: Jade Marks 2007
On April 6, 2006 , one of the most recent tragedies occurred in relation to the carbon dioxide emissions surrounding Mammoth Mountain . Three members of the Mammoth Mountain Ski Patrol died when they fell 21 feet into a carbon dioxide-filled snow cave created by a fumarole. The patrollers were identified as Charles Walter Rosenthal, 58, who lived in Sunny Slopes; John Scott McAndrews , 37, of Bishop and James Jinkuk Juarez , 35, of Granada Hills California, stated Mammoth Local (Shirk 2006, 1). The fumarole which resulted in this tragic accident was a well known vent, which puts out an average of six tons of carbon dioxide per day. The ski patrol team was sent to repair fencing around the fumarole to help keep skiers in the area away from its dangers. During their repairs, the snow collapsed beneath them revealing a snow cave that had been carved out by the fumarole's warmth (Shirk 2006).
Human accidents involving carbon dioxide emissions are not limited to the western United States . Similar volcanic activity occurs through out the world, and has greatly affected many nations and peoples. In 1986 in Cameroon , West Africa, over 1,700 people died when carbon dioxide gas, stored deep in the lake of Nyos , rose up and spread out over the surrounding area (BBC 1). While the cause of such a sudden surge is widely debated, it is certain that all casualties were a result of asphyxiation by carbon dioxide. Some scientists believe that the gas was released when an underwater volcanic eruption occurred. Others claim that the cause of the uplift was a slightly less catastrophic incident, such as a landslide or earthquake, however, no evidence exists to prove these events took place. The most widely accepted theory is that heavy rainfall in the region resulted in a density imbalance in the water of Lake Nyos . As the water naturally re-positioned itself, the carbon dioxide gas was moved to the surface. Today a filtration system has been installed to assure the circulation of water and to prevent the build up of gas that would cause another tragedy ( Lake Nyos 1986).
In 1986, a catastrophic release of carbon dioxide gas from Cameroon 's Lake Nyos (top) asphyxiated thousands of people and animals, including livestock (bottom). The rare natural disaster stemmed from basic geological, physical, and chemical processes associated with some lakes.(Jones 1)
Monitoring The Risk
In response to recent activity the United States Geological Survey and other local organizations have devised a precautionary plan for detecting and notifying affected populated areas of eminent dangers. Beginning in 1982, following a swarm of seismic activity in Long Valley Caldera, a program was established using reducing-gas sensors to monitor gas emissions in the region. These sensors were first used to monitor geochemical activity at Mount Kilauea in 1973. Reducing-gas sensors are based on the relationship between hydrogen and oxygen in water vapor and consist of a thin membrane which releases hydrogen on one side and oxygen on the other. A DC voltage is applied across the catalytic membrane and electric current is allowed to flow in the presence of water; the reaction is reversible. If hydrogen, or other fuel, is supplied to one side of the membrane, and oxygen to the other, a current is generated (McGee 2). A oxygen tank supplies the oxygen for the reaction and the fumarole, vent, or seep provides the hydrogen. Gas molecules diffuse through the teflon sheet inside sensor and are absorbed by the catalytic surface of the electrolyte membrane. Ions created in this process then react with similarly dissociated oxygen molecules. According to the USGS, these sensors respond to a number of gasses including H 2 , H 2 S, SO 2 , CO, COS, HCl, and HF (McGee 2).
There are two measures by which CO 2 can be quantified: concentration and mass flow rate or flux (Farrar 1999). Carbon dioxide concentrations in this region were first determined by using a gas chromatograph set up in various central locations. From these points soil samples were collected, after a steel pipe was driven to the specific depth and the stale gas was purged from the teflon tubing by removing at least two volumes of gas Farrar 1999, 6). According to a USGS report, the CO 2 flux was determined using closed-dynamic-accumulation chambers in which the rate of change in concentrations was measured (Farrar 1999, 6). One report states that, the CO 2 flux was determined using closed-dynamic-accumulation chambers in which the rate of change in concentrations was measured. In 1996 another test was done in which an infrared gas analyzer received gas pumped in a continuous loop from and back into the chamber. The flux of these readings was calculated using a best-fit linear equation. Two monitoring wells were also drilled in the area to learn more about variations in concentrations in the unsaturated zones and the chemistry and isotopic composition of the groundwater (Farrar 1999, 6).
United States Geological Survey workers monitoring gas emissions at Horseshoe Lake Tree-Kill Zone. (Sorey 2000)
In order to ensure safety in such an unpredictable geological setting, the US Geological Survey has created a five-level status ranking response system for Long Valley Caldera, California (including Mammoth Mountain ). Established in 1980, this response system rates the likelihood of volcanic activity on an A-E alphabetical scale, on which A represents an imminent threat and E signifies minimal unrest (Hill 1991). At levels E, D, and C procedure includes contacting the USGS, regional Forest Service and the California Office of Emergency Services, who will then determine what precautionary measures are necessary and are responsible for informing local authorities. When faced with a status A or B alert, a vast number of personnel and resources will be called in to facilitate immediate temporary emergency facilities. An Event Response and Geological Hazard Warning will go into affect in the region (Hill 1991). Currently, an eighteen seismic station network monitors Long Valley Caldera from the inside and the adjacent area, while 38 more stations have been erected within 50km of the caldera. Two dilatometers, seven borehole tiltmeters, one long-base tiltmeter, and three differential magnetometers complete the network (Hill 1991). Consequently, probability has greatly increased that any new volcanic activity will be detected before harm can come to any nearby populated areas.
Potential Benefits
Despite the dangers of gas seeps caused by volcanic activity, there are a number of benefits associated with their existence and evolution. The first of these benefits is volcanic activity prediction. Changes in the location of gas seeps may indicate movement along a fault, movement of the magma body which produced it, or an evolution in viscosity and gas concentration of the magma itself ( Farrrar 1999). At Mammoth Mountain , a region known for attracting many tourists, skiers, and snowboarders, any warning prior to an eruption or seismic activity, could me the difference between life and death.
Many volcanic gases, especially sulfur compounds escape to the surface through fissures and fumaroles that are superheated by intruding magma. These areas are also the locations of compressed superheated water that can be pumped out of their reservoirs and used to generate hydrothermal power. In this respect, volcanic gases can prove as a good indicator of where economically beneficial drilling areas are located. Another important feature of volcanic gas anomalies is their ability to support microbial life. Recent hypotheses suggest that life on Earth may have begun when conditions on the surface were still inhospitable, originating instead in under-water fumaroles. As one Harvard study explains, volcanic gases represent some of the most likely environments in which the precursor molecules necessary for the origin of life were synthesized. The existence of an active, abiotic, organic chemistry in such settings today is fundamental to our understanding of the early Earth (The Organic 2005). Both Yellowstone National Park and Cerro Negro, a young basaltic cinder cone belonging to the Central American Volcanic Belt are currently being studied to determine the validity of such hypothesis. In addition to their extremely high temperatures, many of Yellowstone 's geothermal features exhibit other environmental extremes such as extremely acidic or alkaline waters, or extremely high sulfur or calcium carbonate content, states one survey (Yellowstone 2006). These geothermal areas which vary from site to site contain one of the planet's greatest concentrations of thermophilic biological diversity, including many species that have yet to be identified (Yellowstone 2006). With such diversity of life, fumaroles present a wealth of scientific knowledge.
Conclusion
Volcanic gasses are a complex natural phenomenon that are not confined only to eruptions. Volcanic gasses are being released into the atmosphere every day through fumaroles, gas seeps, and anomalies. These often occur in relationship to seismic and volcanic activity in a given region and can be both hazardous and beneficial to mankind. Mostly comprised of sulfur based gasses, or carbon dioxide these areas are known for killing large quantities of vegetation through asphyxiation of the root system. People and animals trapped in low areas with poor ventilation may also fall victim to asphyxiation. Despite these dangers, the location of fumaroles and gas seeps can provide clues to both impending volcanic or seismic activity, as well as the locations of valuable hydrothermal deposits. In order to ensure the safety of patrons, populated regions, such as the famous Mammoth Mountain , are under close surveillance by the United States Geological Survey. Here, sulfur and carbon dioxide emissions are monitored and a hazard alert system has been established to warn nearby cities and resorts of impending volcanic activity. With such precautions, mankind can continue learning about the unique environments created by fumaroles and gas seeps, and perhaps even discover more about the origin of life on Earth.
Horseshoe Lake Tree-Kill Zone. Photo Credit: Jade Marks 2007
Works Cited
1986: Hundreds gassed in Cameroon Lake Disaster . On This Day. BBC News. < http://news.bbc.co.uk/onthisday/hi/dates/stories/august/21/newsid_3380 00/3380803.stm >
Farrar, Christopher D., John M. Neil and, James F. Howle Magmatic Carbon Dioxide Emissions at Mammoth Mountain , California . United States Geological Survey Water-Resource Investigations Report 98-4217. Sacramento , California 1999.
Hill, P.D., M. J. S. Johnson, J.O. Langbein, et al. Response Plans for Volcanic Hazards in Long Valley Caldera and Mono Craters Area, California . US Department of the Interior, US Geological Survey. Open File Reoprt 91- 270. 1991
Jones, William W. Lakes: Chemical Process Forum . Water Encyclopedia. < http://www.waterencyclopedia.com/Hy-La/Lakes-Chemical Processes.html > . 2007
Lake Nyos 1986 . < http://www.geology.sdsu.edu/how_volcanoes_work/Nyos.html >
McGee, Kenneth A. and A. Jefferson Sutton. A Detailed Study Of The USGS Reducing-Gas Sensor With Field Tests At Long Valley , California . United States Department of the Interior, Geological Survey. Open File Report 90-61.
Shirk, George. Mammoth Ski Patrol Tragedy . Mammoth Local: The Independent Voice of the Eastern Sierra. http://www.mammothlocal.com/news/3_ski_patrollers_die.php
Sorey, Michael L., Christopher D. Farrar. et.al. Invisible CO 2 Gas Killing Trees at Mammoth Mountain , California . United States Geological Survey Fact Sheet 172-96. version 2.0. Revised June 2000.
The Organic Chemistry of Volcanoes: Case Studies at Cerro Negro, Nicaragua and Oldoinyo Lengai, Tanzania <http://adsabs.harvard.edu/abs/2005AGUFM.B23D..04T > American Geophysical Union . 2005
Volcanic Activity. Part II. Chapter 8. Hazardous Earth Processes. Classroom Resource. Adobe Reader. p. 205-231.
Yellowstone National Park . Microbial Life Educational Resource. Science Education Research Center; Carleton College. http://serc.carleton.edu/microbelife/extreme/extremeheat/yellowstone.html November 30, 2006