Geology and Natural Heritage of the Long Valley Caldera
Volcanic Gases: Can’t live with some of them,
may not have existed without them
Gerald Robison
Abstract:
The hazards of volcanoes are many and one of them is the gases that are produced , before, during and after eruptions. In describing the more commonly known hazardous gases, their properties and the how they are formed and produced a basic understanding of the problem volcanoes cause. Different scenarios of gas releases are explored, from fumaroles and vents, gas flux from the ground, limnic deep water lake discharges and volcanic. Understanding the problem helps to mitigate the hazards.
Introduction:
When most people think of the hazards that are associated with volcanoes they think of lava flows destroying houses and great eruptions billowing “smoke” high into the atmosphere. What is not commonly known is the driving force behind the lava flows and eruptions; that volcanic gases control how magma behaves from the time it is formed deep in the earth's mantle to how it arrives at the surface and begins anew as lava. The various gases that are produced in a volcano are released in a number of ways, some harmless, but a good many have a detrimental effect on the people, animals and land near and distant from the volcano. This paper will explain how magma propagates with some of the most commonly produced gases and the hazards associated with the gases. One of the gases, carbon dioxide, will be investigated further in hopes that the reader will have a better understanding of the nature of volcanic gases, and the potential disasters that may happen in the future and the disasters which have already occurred.
Properties of gases
Gases by volume are a small portion of the magma accounting for 1% of a non-explosive or effusive basaltic magma to 7% in an explosive rhyolitic magma (Francis et al, 2004) Gases are under tremendous pressure because of the depths of up to150 kilometers below the earth's crust. Carbon dioxide is dissolved into the surrounding area. As the magma rises, due to its lesser density compared to the surrounding country rock, the pressures holding in the gases are lessened and they are exsolved or released. Nearing the crust, one cubic meter of very viscous magma (rhyolitic) would expand to 670 cubic meters when it reaches the surface and erupts. (Bardintzeff et al, 2000) That 1x1x1 meter cube expands to almost a 9x9x9 meter cube.
Types of Gases:
The most common gas found in volcanic eruptions is water (H2O); estimates exist as high as 99 % of the gas dissolved in magma is H2O. (Bell, 2003) Most sources of the H2O can be meteoric or groundwater that seeps down through the earth via tectonic faults and fractures and natural porous formations like sandstone and limestone. Another pathway is by subduction zone where water laden strata of an oceanic plate subducts under another tectonic plate. (Bardintzeff et al, 2000) The dissolved gases will remain in the rock or magma until it moves to a lesser pressure zone.
Following water is carbon dioxide (CO2). The source of CO2 is thought to have come from sedimentary limestone layers that seeping water has passed through; under extreme pressure CO2 is highly soluble. Next in volume is sulfur dioxide (SO2) and ending with smaller amounts of Hydrochloric (HCl) and Hydrofluoric (HF) acids. There are many other gases in trace amounts. (Mike Sorey et al, 1999)
Effects and hazards of gas release
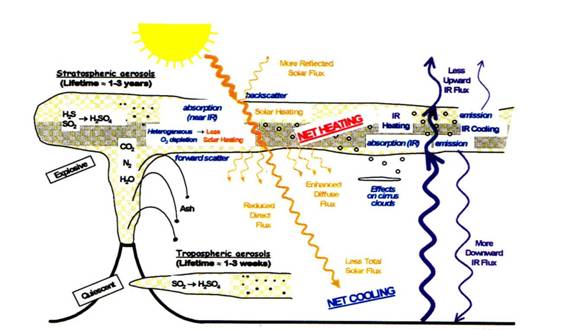
During an explosive eruption ash columns containing various gases have risen up to 40,000 feet. The below cartoon (Figure 1) depicts how a volcano disperses ash & gases. Sulfur and hydrogen sulfides emitted during eruption travel into the stratosphere and chemically react to form acids that are water soluble. These gases react with water vapor and the sun's ray to form an aerosol that can stay afloat for years.
One of the sulfide gases is hydrogen fluoride (HF), which attaches to volcanic ash and falls to earth coating vegetation and contaminating the water supplies. Livestock that ingest the poisoned water and vegetation develop fluorisis, a bone disease which usually results in the death of the animal. Its corrosive properties also damages unprotected metals, similar to HCl acid rain. (USGS, 2000)
In addition, a large ash cloud laden with sulfur dioxides (SO2) in the stratosphere also contributes to the cooling of the earth's surface. The cloud will reflect some of the sun's solar energy back out to space and it will diffuse what gets through, resulting in a reduction of solar energy hitting the ground. The cloud gains some of that heat. Radiating heat leaving earth is also captured by the cloud and gains more heat. The aerosols will also increase cirrus cloud formation with heavier formations of nuclei sites. This then increases the albedo or reflection of the clouds, inducing further heat gain in the stratosphere and cooling the earth's surface. Large eruptions like Mt Pinatubo can alter the global temperatures and enhance upper ozone layer decay. An eruption larger than Pinatubo has catastrophic results. (Sorey et al, 1998)
Figure 1 Schematic diagram of volcanic inputs to the atmosphere and their effects. Alan Robock (2000)
Carbon dioxide: where did it come from?
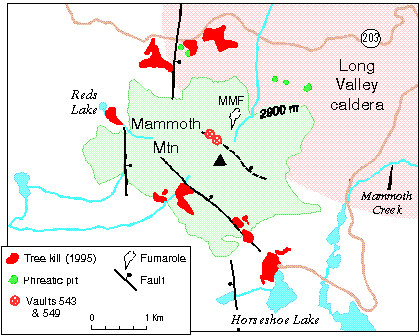
Carbon Dioxide is a colorless, odorous gas that is heavier than air and in concentrations it is lethal to humans, animals and vegetation. Mammoth Mountain (MM) is a unique cumolo volcano (very viscous, multi-eruption layers similar to Lassen's Peak) at the southeast edge of the Long Valley Caldera in central California . MM is as active in venting CO2 as a small eruption of Mount St Helens . Although MM is considered dormant, this venting of an unusual amount of CO2 almost moves it status up to an active volcano. (Sorey, 1999) The genesis of this is believed to be a liquid and gaseous reservoir over an intrusive magma dike approximately 2 km below the mountain. Research by Sorey and Mack Kennedy for the USGS as a result of tree kills on MM had determined CO2 was the cause of the tree kill. Normal CO2 readings for soils read about 1% and findings were in the high 90's for the tree kill areas. The CO2 was starving the trees, denying them the nutrients through the root systems. (USGS Fact sheet 172-96)
Figure 2 Mammoth Mtn tree kill area and known faults
California Geology (1999)To determine the exact source gas, samples were taken from numerous locations (see Figure 3 above) in and around the mountain. Locations included the seven tree kill areas as well as live tree stands, known fumaroles, streams and lakes and man made vaults for mountain snow maintenance. There are two principal sources of volatile gases in magma, 1) the “juvenile” source deep in the mantle through subduction of tectonic plates with its hydrated sediment and rocks and 2) the shallow crust where meteoric water seeps down through porous rocks and faults.
To determine the source, Kennedy, a member of the Berkeley Center for Isotope Geochemistry, used the Helium 3 isotope monitoring method. The nature of CO2 readings are not reliable due to possible multiple sources during its rise to the surface. Trace helium isotopes are a better source determinant with two isotopes, 3He and 4He. The first source is primordial, and not being newly produced, is found deep in the mantle. The latter is constantly being produced through radioactive decay in the crust. There is a high correlation between the two helium isotopes and their origins with the two principal magma origins, therefore providing a much more reliable testing method. Kennedy's results confirmed a higher ratio of 3He, almost 7 times the air ratio at both the fumaroles and the tree kill area. This pointed to a magmatic source. It was also noted that the tree kill areas were associated with the known faults in the area, providing an easy path for the CO2 to travel up.
The amount of CO2 being released is much higher than the theorized magma chamber below the mountain, possibly indicating the reservoir of liquid CO2 capped by a gaseous reservoir under a sedimentary boundary. The 1989 earthquakes may have generated a dike through the sedimentary layer and caused the increased CO2 emanations. (See Figure 3, lower right cartoon, page 8)
Photo 1 Tree kill area below Horseshoe lake. Looking s.e. from summit of Mammoth Mtn. Photo taken by author on 5.16.2006.
Carbon dioxide, the unseen killer
John Rupp, Indiana University, Geological Survey, states that during snowy seasons large trees act like heat conductors and keep the ground surrounding the base snow free. (Personal communication 2006) After a continuous and large snow fall, a well surrounding the base of the tree will develop and trap any CO2 being released through the ground. Wells have been observed as deep as six feet and two to three feet more in diameter surrounding the tree. (Personal observations, May 2006) By disturbing the unstable snow around the well, someone may fall into a highly concentrated CO2 pit and being overcome in seconds. (See Figure 5, p.11 for effects of hazardous concentrations) An example (Photo 1, below) of a cabin similar to that in which a Park Forest ranger almost lost his life accessing it for shelter during a blizzard in March of 1990. (IVHHN, Sorey et al, 1998) In this case, the CO2 can accumulate above the snow line in a tightly secured cabin.
Photo 2 Cabin near Twin Lakes , Mammoth Mtn area. Photo taken by author on 5.16.2006
Mammoth Mtn ski patrol killed by CO2
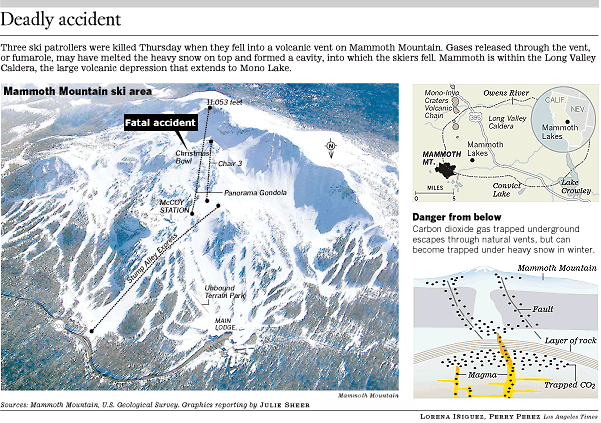
A recent incident on Mammoth Mountain illustrates the danger of volcanic gases. On April 6th, 2006, three ski patrollers lost their lives in a snow cave created by a known fumarole on the north slope of the mountain. This area is in the middle of a popular ski slope and had been roped off. Due to a heavy snow covering the warning fence, it was being relocated when two of the ski patrollers fell through. A third patroller tried to rescue them and was also overcome by the fumes. A second rescuer was also overcome by the fumes, but was pulled out by others.
Figure 3 Picture and cartoons taken from newspaper article in the LA Times, April 7, 2006
Photo 3 Photo of fumarole cave-in where three ski patroller employees fell in and were asphyxiated from CO2 gases. The fumarole area covers about 50/m 2. Mammoth Local, April 7, 2006
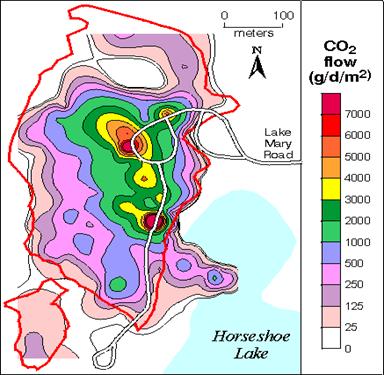
Figure 4 Carbon dioxide flux map, USGS (1999)
Figure 4 shows an area of dead trees on the south side of Mammoth Mountain . The various color shaded areas show different levels of CO2 being released (flux) through the ground. The legend shows areas colored red with highest flux and surrounded by a declining regions of lower flux. The arm of high flux at a neck in the lake are collapse pits containing high CO2 levels that form each fall. The lake was tested and no abnormal readings were found. Bottom silt deposits are theorized to have formed a barrier to the migration of CO2 into the lake water. The measured flux at times was 1,100 tons/day displacing most of the air at ground level (60% or higher). Bardintzeff et al, (2000) The average flux before the tree kill was 6 tons/day. (Hill, USGS, 1999) "The boundary between air and lethal gas can be extremely sharp; a single step upslope may be adequate to escape death.” (Unknown, USGS)
Exposure limits
(% in air)Health Effects
2-3
Unnoticed at rest, but on exertion there may be marked shortness of breath
3
Breathing becomes noticeably deeper and more frequent at rest
3-5
Breathing rhythm accelerates. Repeated exposure provokes headaches
5
Breathing becomes extremely labored, headaches, sweating and bounding pulse
7.5
Rapid breathing, increased heart rate, headaches, sweating, dizziness, shortness of breath, muscular weakness, loss of mental abilities, drowsiness, and ringing in the ears
8-15
Headache, vertigo, vomiting, loss of consciousness and possibly death if the patient is not immediately given oxygen
10
Respiratory distress develops rapidly with loss of consciousness in 10-15 minutes
15
Lethal concentration, exposure to levels above this are intolerable
25+
Convulsions occur and rapid loss of consciousness ensues after a few breaths. Death will occur if level is maintained.
Figure 5 Health effects of respiratory exposure to carbon dioxide. IVHHN (Baxter, 2000)
CO2 and volcanic lakes, the hazards escalate.
There has been recent history of volcanic CO2 killing larger numbers of people and livestock. CO2 in extremely large concentrations at the bottom of volcanic lakes is a relatively new found phenomenon. Lakes Nyos (1986) and Monoun(1984) located in Cameroon , Africa are prime examples of pressurized volcanic CO2 accumulating at the depths of the lakes and exploding violently at the surface, creating huge clouds of carbon dioxide. Lake Nyos ' death toll was approximately 1700, with countless livestock lost and Lake Mounoun was 37 deaths. The trigger that releases the CO2 here was not volcanic, although that is the source of the gas, but “limnic eruptions” or localized lake-over-turn.
A detailed study of limnic eruptions were conducted by Youxue Zhang from the University of Michigan in 1996. It was determined that four conditions need to be in place: deep water of approximately 100 meters, a continuous supply of gas, high solubility of the gas (CO2 is 45 times more soluble in water than air) and accumulation at the bottom of the lake without seasonal lake turn-over. As CO2 saturates the water, it becomes denser and sinks to the bottom of the lake, stratifying and stabilizing. But as the concentration reaches its saturation point, the lake becomes unstable and any small disturbance will cause the gases to exsolve and start a chain reaction. As the CO2 exsolves, bubbles rise and expand, pressure is reduced and more bubbles grow. A conduit of gas forms and draws more saturated water up increasing the amount and speed of ascent. These conditions were all present for both lakes, but the trigger was different. Fr Lake Nyos, it was over saturation. For Monoun the trigger was a possible landslide disturbing the lake bottom. .
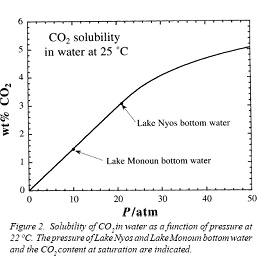
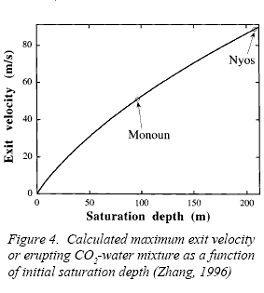
Figure 6 Above graphs taken from Geoscience News, July 1996, Youxue ZhangFigure 6 represents conditions at the bottom of the lakes (left side graph) and at the eruption points. Lake Monoun 's exit velocity translates into a 110 mph column of gas rising 130 meters high, Lake Nyos translates into a 200 mph column rising 400 meters high with a total volume estimated at 1.3 km3 . The heights of the columns explains how livestock were killed on a hill at 110 feet relative to the Lake surface. (Zhang, 1996)
Lake Kivu, D.R. Congo, the next turn-over?
Potentially, the largest concentration of CO2 and Methane (CH4) was recently found at the bottom of a tectonic rift zone lake called Kivu in the Democratic Republic of Congo, Africa. Compared to Lake Nyos , this lake is huge - 1,800 times larger and twice as deep. Analysis of the lake at depths of 350 meters shows an enormous amount of dissolved CO2 and CH4 - 55 billion m3 of methane or enough to power the US for a month and 6 times as much CO2 or 200 km3. (Univ of Bristol , UK Webprojects 2002)
In 1972, Professor Robert Hecky (Univ of Michigan) discovered through sediment samples, that over the past 5,000 years, the lake has had a mass local extinction in and around the lake every 1,000 years, sweeping vast amounts of vegetation into the lake from around its shores. Coupled with the 2002 eruption of Mt Nyiragongo, which did reach the lake but did not overturn it, and its continued volcanic activity, the potential is there for a catastrophic venting endangering the 2 million people that live around its shoreline.
Toba, near extinction of human life
In 2000, Michael Rampino, an environmental scientist and Stanley Rampino, an anthropologist, proposed a theory of near extinction for the human race. Approximately 73,500 years ago Toba, a volcano in Indonesia , erupted in what is considered the largest explosion in the last several 100,000 years. No recorded event was comparable and computer models of a “nuclear war winter” were the closest global analogy. The eruption produced a 35,000 foot ash column, 2800 km 3 of ash fall and 10 15-16 g of hydrogen sulfide (H2SO4) aerosols. The estimated time of suspension was 6 years. Evidence of a rapid cooling of the earths climate were confirmed by contemporary ice cores from Greenland's ice fields, coral reef samples and models of the 1815 Tambora eruption. This also occurred during a glacial period and while not the sole cause of the cooling period, certainly it was a contributing factor.
The result was a global environmental catastrophe. The equator recorded freezing temperatures, tropical forest were decimated, sea levels drastically lowered due to most of the earths fresh water being locked up in the global ice sheets. The global air system dried out, drought and forest fires were the norm. The oceans viability was at a critical point. Finally, human kind was almost wiped out. A bottleneck in human population occurred. It was estimates of a drop in total population of 3,000 to 4,000 over a 20,000 year period with a worst case scenario of a breeding population of 40 to 600 females over a 200 year period. Supporting this severe drop in world's population was a similar drop in chimpanzees during the same period. Volcanic gas almost ended human kind by extinction.
In an ironic conclusion on volcanic gases a group of scientist at the Scripps Research Institute and Salk Institute for Biological Studies in 2004 reported a possible answer to the origin of life on earth, carbonyl sulfide, a volcanic gases. In experiments with amino acids that would have been present on early earth, various forms of peptides were formed. Peptides are the building blocks of proteins for the primitive life forms that developed into photosynthesis producing cells. The theory is in an early stage and is being aligned with a theory by a Russian scientist, A.I. Oparin, who in the 1920's suggested life arose from the violent energy of lightning, solar radiation, comet impacts and volcanic events.
Conclusion
It's obvious that we can not control one of the most destructive forces on earth. What we can do is mitigate the hazards. The CO2 discharges in the Mammoth Mountain and Long Valley Caldera area obviously need constant monitoring. Educating the “visiting public” of the hazards is a start. The awareness and unfortunate death of the ski patrol employees is a very strong start and the local skiers haven't forgotten that a cross-country-skier died on the mountain in CO2 saturated snow well in May of 1998. It reminds us that a skiing playground is also a killing field. The fumaroles on Mammoth Mountain could be cordoned off with a self elevating protective fencing, similar to docks at a marina that rise with the tides. The area involved is about 50 m2 and could set up with sensors that raise the fencing as the snow builds. The supports for the system should be accessorized with self-contained-positive-pressure-full-face-oxygen masks. It also could be rigged with a motorized hoist with harnesses for lower and lifting into the snow caves created by the fumaroles. Continual training for all possible accidents is a must. Patrols should have the oxygen mask as part of back pack in cases of emergency.
The deep water lakes are currently being study for degassing by vent pipes and as an economic resource (methane) as in the case of the Lake Kivu . For the poorer African nations the degassing of the lakes is economically unfeasible, needing an enormous amount of resources and time to degas. If the resources of Lake Kivu are realized some profits and the technology should go to financing of the degassing of other lakes.
References:
1. California Geology, (Sept 1999) Magmatic gas emissions from Mammoth Mountain. Mike Sorey et al.
2. Crater lakes of Java: Dieng, Kelud and Ijen. Excursion guide book, IAVCEI General Assembly, Bali (2000). Manfred J van Bergen et al.
3. Geological Hazards, their assessment, avoidance and mitigation, (2003) Fred G Bell.
4. Geoscience News, July 1996, “Cracking the killer lakes of Cameroon ” Youxue Zhang, Univ of Michigan .
5. Geological Society of America , (2000) Special paper: Volcanic winter in the Garden of Eden : The Toba super eruption and the late Pleistocene human population crash. Michael Rampino and Stanley Ambrose.
6. Journal of Geophysical Research, “Carbon dioxide and helium emissions from a reservoir of magmatic gas beneath Mammoth Mountain volcano ”, Vol 107 no B7, pages 15,303 to 15,323, July 1998, Mike Sorey et al.
7. L.A. Times
http://www.latimes.com/news/printedition/la-me-ammoth7apr07,0,3616279.story8. Mammoth Local (newspaper) article written by George Shirk, April 2006. http://www.mammothlocal.com/news/3_ski_patrollers_die.php
9. Personal communication, John Rupp, May 2006, Indiana University geological field trip to the Sierra Nevada Long Valley Caldera, California .
10. Personal observations by author during geology field trip, May 9-24, 2006, Indiana University, Sierra Nevada and Long Valley caldera area in California .
11. USGS bulletins, fact sheets and websites:
• Response plan for volcano hazards in the Long Valley Caldera and Mono Crater region, California . Bulletin # 2185, David P. Hill etal. (2002).
• Long lasting eruptions of Kilauea volcano, Hawaii leads to Volcanic-air pollution (VOG). April 2000.
• Volcano hazards fact sheet, # 9585
• http://volcanoes.usgs/hazards/what/volgas/volgas.html12. Volcanoes, 2nd edition, (2004) Peter Francis and Clive Oppenheimer.
13. Volcanology, 2nd edition (2000) Jacques-Marie Bardintzeff and Alexander R. McBirney.
14. Websites:
• http://www.chm.bris.ac.uk/webprojects2002/whitehouse/Kivu.htm
• http://www.chm.bris.ac.uk/webprojects2002/whitehouse/Kivu.htm
• http://volcano.und.edu/vwdocs/volc_images/southeast_asia/indonesia/dieng/dieng3.html
• http://www.geo.mtu.edu/volcanoes/hazards/primer/gas.html
• http://volcanology.geol.ucsb.edu/gas.htm
• http://lvo.wr.usgs.gov/unrest.html
• http://www.sciencedaily.com/releases/2004/10/041008024453.htm