Geology and Natural Heritage of the Long Valley Caldera
Carbon Dioxide Emissions at Mammoth Mountain, California
Katherine Neff
According to Dr. David P. Hill and Dr. Roy R. Bailey of the United States Geological Survey, the most common geological question in Mammoth, California is Where is the volcano? Although Mammoth Mountain does not resemble the classically recognized cone-shaped volcano, the mountain experienced significant volcanic and seismic activity in the past two decades, which have brought the mountain the recognition as a potentially hazardous volcano.
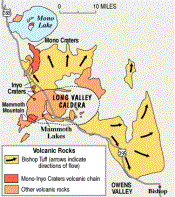
A massive volcanic explosion 760,000 years ago created the Long Valley Caldera in California. A build up of magma beneath the earth's surface caused an uplift of the crust which led to the explosion. Afterwards, the crust sank over a mile, into a depression measuring 10 miles wide and 20 miles long. A magma chamber still exists beneath the caldera. The fumaroles and hot springs confirm the presence of a magma body. The underground magma heats groundwater which rises to the surface and releases in hot springs or steam vents (Hill et al. 2000). Recent uplifting caused the Resurgent Dome in the middle of the caldera to rise 2.5 feet over the past two decades. This volcanic unrest, on a previous stable volcanic system, prompted the USGS to put in place an Emergency Response plan and to closely monitor the region for further signs of unrest (Hill et al. 2000).
Map from: U.S.G.S. Fact Sheet 108-96, version 2.1The Mono-Inyo Crater volcanic chain lines the southwest side of the Long Valley Caldera. Mammoth Mountain, a volcano within the Mono-Inyo chain, formed 50,000 years ago from multiple dacite eruptions (Sorey et al. 1999) Dacite magma is an intermediate between rhyolite and andesite (Kearey 2001). Over the last 5,000 years, eruptions in the Mono-Inyo volcanic chain occurred periodically every 250-700 years. The active volcanic chain is being closely monitored for signs of future eruptions which include earthquakes, uplift of magma and gas emissions (Hill et al. 1998).
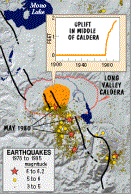
U.S.G.S. Fact Sheet 108-96, version 2.1Starting in the 1980's numerous earthquake swarms took place throughout the Long Valley Caldera concentrated in the southern section of the caldera. In May of 1989, seismic activity started in Mammoth Mountain, a volcano on the southwest edge of the Long Valley Caldera, with a period of earthquake swarms. Scientists collected data indicating that a dike was rising beneath the mountain and intruded 2 km below the surface of Mammoth Mountain (Sorey et al. 1999). The following year, patches of trees died on the side of the mountain. The tree kill phenomenon was not connected with the area volcanism until 1994. Originally, scientists attributed the death of the trees to an infestation or periodic droughts in the area. In 1990, a Forest Service ranger, making a routine visit stayed in a cabin in the Horseshoe Lake area. Since carbon dioxide is heavier than air, it filled inside the cabin. The high concentration of carbon dioxide caused the ranger to experience symptoms of asphyxia. The ranger left the cabin before the symptoms became fatal (Sorey et al. 1998). The normal gas content of air is 0.04% carbon dioxide. Scientists determined that breathing in concentrations of carbon dioxide at or above 30% can quickly induce unconsciousness and cause death (USGS 2000).
Copyrighted photo courtesy of John D. Rogie: U.S.G.S. Fact Sheet 172-96Volatile compounds found in magma evaporate out of the liquid quickly. While dissolved within the magma, the volatile compounds control the viscosity. The exsolved gas, or the degassing process affects how the magma erupts. When the amount of water increases inside the magma, the density decreases because the same amount of magma now has more water than before. Also, the viscosity decreases because viscosity is the resistance of a substance to flow. With more water inside the magma, the magma flows smoother. Water vapor is the most abundant volatile volcanic gas. Water has been calculated as 70-99% of the volume of gas within the magma. The primary source for water in volcanic material is meteoric meaning the water from rain and snow seeps into porous rocks. As the magma rises, it takes in the water from the rocks. Another source of the water in magma is juvenile, meaning the water comes from the subduction zones in the mantle (Bardintzeff 2000).
The second most abundant volatile gas in magma is carbon dioxide. Carbon dioxide in magma originates in the mantle where the magma forms. The carbonate rich metamorphic rocks which the magma passes through can influence the quantity and isotopes of carbon dioxide found within the magma. The total amount of carbon dioxide released by volcanic eruptions each year is 31 million tons. Fumaroles and soil emit another 34 million tons of the gas every year (Bardintzeff 2000). Other gases emitted from volcanoes include hydrogen sulfide, hydrogen, carbon monoxide, hydrogen chloride, hydrogen fluoride and helium. Typically, volcanic gases are emitted from eruptions, but gases can also be released from underground magma through outlets such as the soil, volcanic vents, fumaroles and hydrothermal systems (USGS 2000). In the case of Mammoth Mountain, the dike caused a releasing of volcanic gases (Sorey et al. 1998).
The extent volatile compounds affect the magma depends on different characteristics. First, the concentration of the volatiles in the magma influences how much affect the volatiles have upon the eruption. Basaltic magma averages only 1% overall volume of volatile elements. Andesitic magma contains 6% and Rhyolitic magma contains the most volatile compounds at 7% overall volume. The other variables include the solubility limit and the gas phases (Bardintzeff 2000). Volatile compounds influence eruptions because the presence of volatiles alters the viscosity of magma. A viscous magma is explosive because of the large amount of gas the magma can retain. Once pressure is released from the gas at the surface, the gas expands rapidly causing an explosion. Magma with a lower viscosity and therefore less volatile compounds will flow as opposed to explode (Wheeler 2000).
Photosynthesis is a process that plants use to create energy from sun light. The process requires the intake of carbon dioxide, water and sunlight which the plant uses to release oxygen, glucose and water. Normally, trees would take in carbon dioxide and use the compound to create glucose. The reason the trees at Horseshoe Lake and other areas around Mammoth Mountain died was over saturation of carbon dioxide. The normal gas composition of soil only contains 1% carbon dioxide. The soil readings in the tree kill zones around Mammoth Mountain show carbon dioxide as up to 95% of the gas content within the soil. While carbon dioxide is necessary for the trees vital processes, the trees roots also need to be able to absorb oxygen and nutrients from the soil. With the carbon dioxide gas content so high, the trees were not capable of obtaining the necessary nutrients (Sorey et al. 2000).
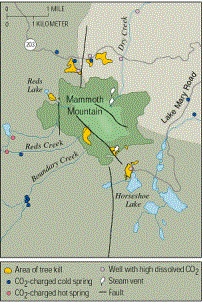
Many areas around Mammoth Mountain have been affected by tree kill including, Horseshoe Lake, Horseshoe Lake Fumarole, Red Creek, Red's Lake, Chair 12 spring, the Main Lodge and the Main Lodge Spring. These various sites are spread out around the mountain on the north, west and south side. All of the areas share in common the adjacency to a fault (Sorey et al. 1998).
U.S.G.S. Fact Sheet 172-96, version 2.0The level of carbon dioxide gas released into normal soil averages 10 grams of gas per day over a square meter. The measurement of the carbon dioxide flux in the tree kill area around Mammoth Mountain ranges from 63 g/d/m2 6,500 g/d/m2. The high level of degassing found at Mammoth Mountain has been recorded at other volcanoes such as Kilauea, Hawaii. Generally, the high level of degassing also included significant emissions of hot gas or a volcanic eruption. Neither the hot gas nor an eruption has occurred at Mammoth Mountain since the degassing began. (Sorey et al. 1999). Instead of hot, Sorey et al. described the gas emissions at Mammoth Mountain as cold CO2 diffuse. Scientists estimated that 30-50 tons per day of carbon dioxide flow through the mountain's cold groundwater system (Sorey et al. 1998).
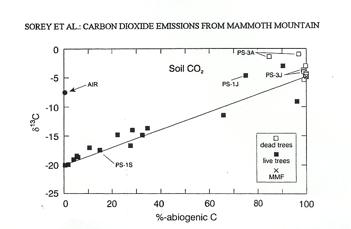
The carbon isotopes found in the carbon dioxide released can show the source of the gas. There are two specific isotopes that scientists examine as an indicator of the gas' origin. The abundance of 13C, a stable isotope, changes depending on biogenic and abiogenic sources. Respiration and organic material decay create biogenic carbon dioxide in the soil. Abiogenic sources include magmas and carbonate rocks. Within biogenic sources the value ofƒÂ13C is - 20 parts per thousand. The delta stands for the difference from the scientific standard while the negative sign indicates that the figure is less than the scientific standard. When isotope 13C is identified among non biogenic source, the ƒÂ13C value is -5 parts per thousand. The other isotope isolated in carbon studies is 14C, a radio active carbon. The half life of 14C is 5,700 years. Therefore, the isotope is found in biogenic sources, but not in abiogenic sources because the half life is short enough that the isotope decays (Sorey et al. 1999).
The data collected at the tree kill sites point to a non biogenic source since no 14C was found and the 13C readings showed ƒÂ13C value as - 5 parts per thousand. There were two sites around the mountain which had a non biogenic origin, but the trees did not die. In each of the places, the carbon dioxide gas content of the soil was only 10%. A reasonable explanation therefore is that the trees could still function normally with those carbon dioxide levels (Sorey et al. 1999).
Graph from Sorey et al. 1998
The Graph shows how the dead trees are surrounded by carbon dioxide from a non biogenic source.In addition to the measurements of the isotopic carbon patterns on Mammoth Mountain, helium isotopes help to clarify the origin of the gas contents. While the amount of 13C isotopes indicated an abiogenic origin, both magma and carbonate rich metamorphic rocks could be the origin. The metamorphic rocks in the area had ƒÂ13C values ranging between 0 to 12 parts per thousand. The magmatic origin of ƒÂ13C - 5 parts per thousand falls within the range of the isotopes within the metamorphic rock. To clarify the origin of the gas, the helium isotopes are used to differentiate between a magmatic and metamorphic rock source. The ratio between the 3He and the 4He isotope is contrasted with the air to find the value RA. The RA value for metamorphic rocks within the Caldera is measured at 0.10 RA. Rocks from a volcanic origin within the caldera have a RA value of 7 RA. The gas emitted from the steam vents at Mammoth Mountain Fumarole fluctuated from 5-7 RA starting in the year 1989. Therefore, the helium isotopes also point towards a magmatic source (Sorey et al. 1999).
The fluctuations in the Helium isotope level on Mammoth Mountain is attributed to the magma intrusions caused by the earthquakes. The variations of Helium levels follow the different seismic activity on the volcano. During the lower seismic activity, the level decreases. After 1989, the RA value increased from around 3 RA to 7 RA during the earthquake swarms. After a decrease in RA value, the values rose during the long-period earthquakes, quakes which take place 10-30 km under ground. The fluctuations imply that the seismic activity and the gas emissions at the surface are interrelated (Sorey et al. 1999).
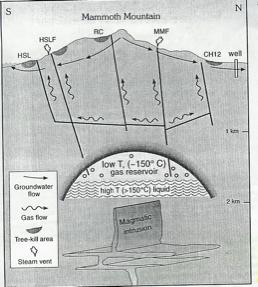
A model proposed by Sorey et al. explains the origin of the carbon dioxide and helium release at Mammoth Mountain. The estimated depth of the model is 2 km below the surface of the mountain, the depth of the dike intrusion in 1989. Above the magma, a high temperature liquid reservoir acts as a buffer. The liquid contains high amounts of carbon dioxide in order to maintain a gas cap. Magma releases reactive gases which have not been detected at the surface such as, sulfur dioxide, hydrogen chloride and carbon monoxide. These reactive gases are stopped by the buffer. Above the liquid reservoir resides a lower temperature gas cap within fractured rock. On top of the gas layer an impermeable or low permeability cap prevents the gas from escaping. The faults and fractures within the layer allow the gas to flow through the area of low permeability. In 1989, following the dike intrusion or the recent seismic activity, more gas was released through the area of low permeability. After escaping through the seal, the low-pressure gas was free to spread laterally and vertically away from the point(s) of leakage along flowpaths that were not fluid-filled, or at least that the flow was not impeded by interaction with a thick-saturation zone (Sorey et al. 1998)
Image from Sorey et al. 1999
The diagram shows the model of the dike intrusion at Mammoth Mountain. It also shows the faults through the reservoir up to the tree kill areas.Gas has been released at Mammoth Mountain since 1989. The dike of 1989 was estimated by scientists to range between 0.01 km3 and 0.04 km3. The fact that gas continues to be released over the past two decades supports the theory of a gas reservoir underneath the mountain. The gas reservoir is theorized to hold the gas degassed from the magma and collect the gas which would be released from high carbonate metamorphic rocks heated up by the magma intrusion (Sorey et al. 1999).
Degassing of magma may indicate the start of a future eruption. Although Mammoth Mountain is experiencing magma degassing, it does not necessarily imply that the volcano will erupt. Sorey et al explains It seems most likely that the current high gas flux results from fracturing and breaching of the seal on a large gas reservoir during dike emplacement in 1989 and that this reservoir has existed beneath Mammoth Mountain for a considerable period of time. Whether or not the volcano will erupt does not depend on the degassing, though the presence of a gas reservoir will increase the explosiveness of an eruption. Other indications of volcanic activity include seismic activity and uplift of magma. Scientists continue to closely monitor Mammoth Mountain for gas flux, earthquakes and upwelling (Sorey et al. 1999). While eruptions are unpredictable, one thing is for certain; with all of the volcanic activity over the past two decades, Mammoth Mountain has established itself as a volcano.Works Cited
Bardintzeff, J.M. and A.R. McBirney. Volcanology, Second Edition. Sudbury, Jonen and Barnett, 2000.
Hill, David P. and Dr. Roy R. Bailey. The Big Eleven: Mammoth's 11 Most Asked Geologic Questions. Mammoth Times.Hill, David P. et al. Future Eruptions in California's Long Valley Area-What's Likely? USGS Fact Sheet 073-97. U.S. Department of the Interior, U.S. Geological Survey. November 1998.
Hill, David P. et al. Living with a Restless Caldera-Long Valley, California. USGS
Fact Sheet 108-96, version 2.0. U.S. Department of the Interior, U.S. Geological Survey. May 2000.Kearey, Philip. The New Penguin Dictionary of Geology, Second Edition. Penguin Books Ltd: London, 2001.
Sorey, Mike et al. Carbon Dioxide and Helium Emissions from a Reservoir of Magmatic Gas Beneath Mammoth Mountain, California. Journal of Geophysical Research, Vol 103.No. B7, pp 15,303-15, 323. July 10, 1998.
Sorey, Mike et al. Invisible CO2 Gas Killing Trees at Mammoth Mountain, California. USGS Fact Sheet 172-96, version 2.0. U.S. Department of the Interior, U.S. Geological Survey. June 2000.
Sorey, Mike et al. Magmatic Gas Emissions from Mammoth Mountain. California Geology. Sept./Oct. 1999.
U.S. Geological Survey, Long Valley Observatory Website. Volcanic Gases and Their Effects. U.S. Department of the Interior, U.S. Geological Survey. 15 Feb 2000. <http://volcanoes.usgs.gov/Hazards/What/VolGas/volgas.html>.
Wheeler, Mark. When Magma is on the Move, Smithsonian. Feburary 2000. pp 40-48.