Geology and Natural Heritage of the Long Valley Caldera
Hydrothermal Alterations and the Formation of Metal Ores in the Sierra Nevada's
Megan Elizabeth Patterson

The study of mineralogy and ore deposits can at large be attributed to hydrothermal alteration processes where the chemical alteration results in many metallically rich chemical compositions: gold, quartz, tin, etc. This hydrothermal alteration process mainly combines tectonics, volcanism, and heated water. The basic mineralogy of rock is altered as a result of condition changes in the temperature, pressure, or chemical composition/makeup. This has shaped things such as the California gold rush and has been an economic boom since its discovery.
The Hydrothermal Alteration Process
Hot water or “hydrothermal fluids” pass through a nearby igneous rocks fractures or porous spaces within the rock, altering the chemical composition (Adams). This chemical alteration can be the result of “adding, removing, or the redistribution of the chemical components” (Adams). These chemical components I have mentions entail the basic makeup of the rock. For example, the chemical composition of kaolinite is (Al4Si4O10 (OH)8) according to the “Alteration Chemistry” handout distributed in the classroom materials, while the chemical composition before it was altered is KALSI3O8 + H2O.
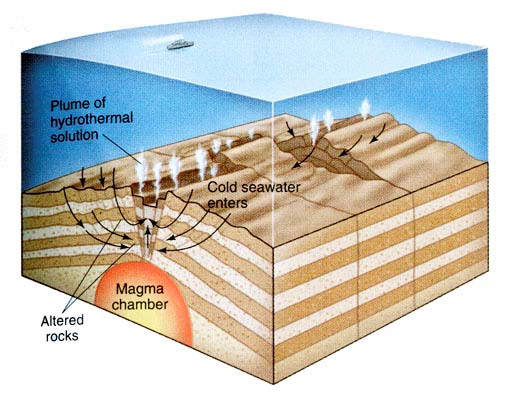
An example of a hydrothermal system and its circulation. From “The Blue Planet” by Brian J. Skinner (1995). Also taken from Williams, Curtis “Hydrothermal Alteration and Mineral Deposits.” (2002).Typically, these “hydrothermal fluids” or “aqueous solutions” carry many metals in addition to “silicates and other non-metallic materials” (Jones and Hutton). The different igneous rock compositions can range in a variety of minerals, when the water that is heated by a nearby magmatic chamber rises in temperature and alters the nearby igneous rocks the hydrothermal solution then becomes mineral rich. This mineral-rich solution rises, meandering its way through fractures or cracks in the rock cooling as it moves and dissolving other minerals on its path, once this solution has cooled in the fracture of the rock- creating veins.
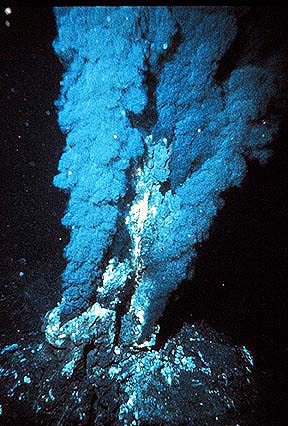
Black smoker from the mid-Atlantic Ridge This is an example of hydrothermal alteration releases in the ocean.The energy behind the hydrothermal alteration process is the “geothermal cell” (Jones and Hutton 2000). A “geothermal cell” is the place in which the water is heated (Jones and Hutton 2000). Typically, from the source the cold water moves through the fractures and cracks within the rock until it is heated. As stated earlier, it is heated by a nearby magmatic chamber. The heated water solution then passes through the rocks dissolving metallic ions and other minerals, therefore altering the chemical composition and makeup of the rock.
Typically, the hydrothermal solutions have a high saline content; therefore the movements of these fluids alter the rock. With the minerals varying conditions: temperature, pressure, pH and Eh if the conditions change a condition will therefore change. This may then cause the rock to react with nearby materials. According to Jones and Hutton “the temperatures at which the minerals are formed range from 50 to 650°C.” These highly varying temperatures create for a highly conducive altering agent. “The movement of these hydrothermal fluids in the crust of the Earth is known as “hydrothermal convection.” (McCaffrey and Pavey). “The reasoning behind this terminology is the root meanings of the word: hydro meaning water, thermal meaning heat and convection meaning transfer of heat by physical movement of material” (McCaffrey and Pavey).
Hydrothermal Ore Formation
An ore is a rock that is rich in the metals, often times metal. The formation of hydrothermal ores is attributed to the hydrothermal solution or fluids that filter metals and minerals from the rock. These metals are then deposited into fracture and cracks within the rock called: veins. Fractures are a result of things such as water freezing and expanding. Fractures can be caused by seismic activity, when the ground shifts fractures and fissures are left in the bedrock and other components that make up the crust. The continental crust is composed of granitic rocks, whereas the oceanic crust is composed mainly of basaltic rock.
Click to see an illustration of the basic principle of metal concentration by hydrothermal processes
This animated figure has been taken from (McCaffrey and Pavey).
Click to see an illustration of the various features needed to form a hydrothermal ore deposit
This animated figure has been taken from (McCaffrey and Pavey).
Some necessary factors for development of hydrothermal ore deposition include: water source, source of ore components, and transport of ore constituents, permeability, cause, and ore deposition. Each of these factors strongly influences the hydrothermal process.
The main sources of water which will eventually move to a place to where it will be heated by the nearby magmatic chamber include: “surface water, including groundwater, referred to as meteoric waters; sea water; connate water or water enclosed in the rocks at the time of formation; metamorphic water; and, magmatic water (from magma)” (Jones and Hutton 2000). Meteoric waters can be collected from precipitation factors such as snow, rain, etc; whereas, “formation water that has been trapped with a specific area; such as pores of sediments”(McCaffrey and Pavey). This water will eventually become highly concentrated with minerals and metals that it dissolves as it moves through the igneous rocks.

“Figure 16.24b A magmatic ore deposit. Layers of pure chromite (black) enclosed in layers of plagioclase, settled out during the crystallization of the bushveld igneous complex. This unusually fine outcrop is located at Dwars River in South Africa” www.usd.edu/esci/figuresOre metals are commonly derived from Earths crust. In addition to the already available source of ore constituents is the high salinity that is assists the transport of the ore constituents. The hydrothermal fluids, as a result of the highly concentrated minerals (Cl, F, and CO2), easily carry the highly concentrated metals. The wasy sin which these metallic ions are typically transported is through “complex ions” (McCaffrey and Pavey). “A complex ion is defined as a single chemical species made up of an unusual combination of two or more atoms” (McCaffrey and Pavey). These complex ions prevent deposition of the metal during the move. In contrast, the simple ions easily loose the metal during the transport.
This process can only happen if the solution can move through the rock; therefore permeability plays a key role in the movement of the hydrothermal solutions. These solutions can move through passageways such as pores, cracks, fractures, etc. It is essential for the success of hydrothermal alterations. Deposition of the ore minerals is usually due to (1) temperature decrease- cooling, (2) decrease in pressure, (3) change in composition of hydrothermal solutions. A temperature decrease is a result of cooling of the hydrothermal fluids. While a decrease in pressure can be the result of many factors.
Concentrations of these metals in a constrained space equivocates an ore. Deposition of the ore can be sub aerial, seafloor, found in a fracture, or a rock. Hydrothermal faulting may develop breccias and gouge. This is a form of mineralization and alteration that takes place where there is a wealth of fine grained veinlets. Mineral zoning patterns often times develop near ore deposits as a result of changes in temperature, the chemical composition of the fluid, and gas content.

“Figure 16.23 Ore formed by metamorphism. Ore of the Tem-Piute Mine, Nevada. White is calcite, purple is fluorite. Ore minerals visible are sphalerite (brown, lower left), pyrite (gold) and scheelite (CaWO4), the sugary pale brown mineral upper left and lower right. Scheelite is an important tungsten ore mineral” www.usd.edu/esci/figures
Vein and Skarn Deposits
Veins as mentioned several times above are the most common fashion in which the hydrothermal concentrated material cools. Hydrothermal ore is formed when the cracks, faults, and fractures are filled. Most commonly they appear in volcanic arcs and collision terrains. The reason behind this is that the magmas circulate the fluid moving combining with the additional stress resulting in a major fracture. The fracture is then filled with the hydrothermal solution cooling at some point. The metals found in the veins are typically found in the crust and maybe the source of the metals.
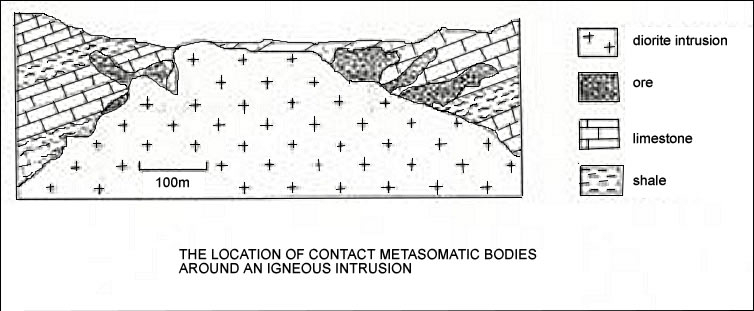
”Vein and disseminated chalcopyrite in quartzite” figure taken from www.zambia-mining.com/ gold%20vein.jpgThe large skarn deposits form as a result of fluid replacing the rock. Often times the rocks are made of limestone.
(McCaffrey and Pavey).
Epithermal Deposits

Figure taken from http://www.davidkjoyceminerals.com/graphics/841.jpg6.1
Gold is known as an epithermal deposit because it is found at a very shallow depth. With ore deposits there are two types of classifications: principal and secondary. A primary ore is made primarily of one main metallic component. Gold is an example of a primary ore because it is made primarily of gold; however have components such as silver can be found. Another classification is a gangue mineral. Gangue minerals generally include quartz, calcite and other such minerals as kaolinite and chlorite. Gold is thought to precipitate from groundwater near in areas nearby a hot spring. These deposits are commonly found in volcanic arc regions such as the Sierra Nevada region.How this all relates to the Sierra Nevada Region
While visiting beautiful areas such as Blue Chert and the Kaolinite mill I was intrigued by how heated water could chemically alter rocks to make such beautiful things. With the seismic activity often causing the faults and fissure for the altered rock to eventually fill; tectonics too has its hand in it. Tectonically the subduction of a plate will force water down to eventually be heated. Often times even the magma is closer to the surface and can heat a greater amount of water. Hot Springs are a great example of magmatic pocket that is closer to the surface; as a result the water is heated and emission of sulfur and CO2 are greater. Other landforms that can be attributed to hydrothermal alterations are the Inyo Craters. The groundwater circulated through the magma as it was rising resulting in steam charged explosive eruptions.
Bodie is another great example of how this process can affect life: people were driven to the adventure and possible money they could find. With the potential money came thieves, prostitutes, and outcasts. This now abandoned town that had been converted into a preserve shares an interesting birds-eye-view to California’s past.
References
Adams, David. Delta Mine Training Center http://www.dmtcalaska.org/course_dev/explogeo/class08/notes08.html
Barnes, Hubert L., Geochemistry of Hydrothermal Ore Deposits, 1 – 13, 303 – 307, 435 – 448, 1997.
Bove, Dana J., Compositional Changes Induced by Hydrothermal Alteration at the Red
Mountain Alunite Deposit, Lake City, Colorado, U.S.G.S. Bulletin 1936.Gonchar, G.G., Fluids in the Crust: Equilibrium and Transport Properties, 1 – 41, 1995.
Geological Association of Canada., Alteration and Alteration Processes Associated with Ore – Forming Systems, 1 – 43, 315 – 339, 1994.
Hagemann, Steffen Dr., Hydrothermal Alteration Systematics, Ore Genesis Lecture Series, Lecture 364, 2001. http://www.virtualgeology.com .Jessey, Dr., Theories of Ore Genesis GSC433 Lecture. http://geology.csupomona . edu/drjessey/class/GSC433/Genesis.htm
Jones and Hutton., University of Wollongong, GEOS102 Ore Bodies 3- Hydrothermal Deposits Lecture http://cedir.uow.edu.au/Projects/GEOS102/lectures/ach6.html . 2000.
Kirkemo, Harold and William L. Newman and Roger P. Ashley. “Gold”. U.S. Geological Survey. << http://pubs.usgs.gov/gip/prospect1/goldgip.html >> 1997.
Lamber, David., The Field Guide to Geology. New York. Facts on File. 1988.
McCaffrey and Pavey, Lecture 1 - Ores and Ore Minerals, 5.2 Criteria for Hydrothermal
Ore Formation, Lecture 6 - Ores formed by Hydrothermal Processes II: Intracrustal Deposits. http://www.dur.ac.uk/juliette.pavey/geology/lectoutline.htmPirajno, Franco., Hydrothermal Mineral Deposits, 22, 33, 42 – 44, 101, 110 – 123, 1939.
Schafersman, Steven D., Miami University. GLG 111 Chapter 21: Geological Resources. Lecture Outline. http://www.utpb.edu/SCIMATH/schafersman/geology/phy-geol/lecture-notes/ch21-resources.html.Skinner, Brain J., The Blue Planet: An Introduction to Earth System Science, 419 – 425.
Williams, Curtis. Hydrothermal Alteration and Mineral Deposits. http://www.indiana. edu/~ sierra/papers/williams.html.
Materials collected from G188 binder. Compiled by John Rupp, Michael Hamburger, and Assistant Instructor. May 10-25, 2003.
Field Work from Sierra Nevada Region, Geology G188. Compiled by Megan Patterson. May 10-25, 2003.
Sites only used for images/ figures
http://www.davidkjoyceminerals.com/graphics/841.jpg
www.zambia-mining.com/gold%20vein.jpg